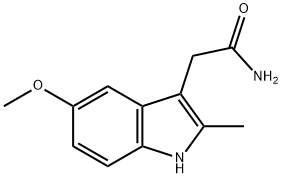
2-(5-methoxy-2-methyl-1H-indol-3-yl)acetamide synthesis
- Product Name:2-(5-methoxy-2-methyl-1H-indol-3-yl)acetamide
- CAS Number:15992-10-6
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25
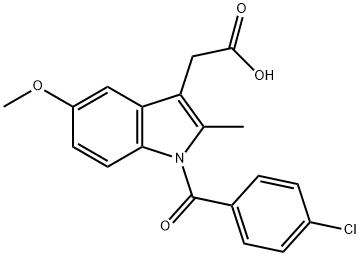
53-86-1

15992-10-6
General procedure for the synthesis of 2-(5-methoxy-2-methyl-1H-indol-3-yl)acetamide from 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetic acid: 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetic acid (14.3 g, 40 mmol) was Dissolve in anhydrous THF (100 mL) and cool to -10°C. N-methylmorpholine (5 mL, 45 mmol) was added slowly dropwise with vigorous stirring. after 10 min, ethyl chlorocarbonate (4 mL, 42 mmol) was added slowly dropwise for 30 min. Subsequently, NH4OH (100 mL) was added and the reaction mixture was stirred for 3 hours at room temperature. Under stirring, 4 mol/L NaOH solution (200 mL) was slowly added until the solid was completely dissolved. The reaction mixture was partitioned and the aqueous layer was extracted with CH2Cl2 (3 x 200 mL). The organic layers were combined, washed with brine, dried over anhydrous Na2SO4 and concentrated in vacuum to give the crude product. The crude product was washed with water to give white solid 2-(5-methoxy-2-methyl-1H-indol-3-yl)acetamide (7.644 g, 88% yield). The melting point of the product was 153-155°C. 1H-NMR (400MHz, CDCl3) δ: 7.91 (s, 1H), 7.20 (d, J=8.61Hz, 1H), 6.91 (d, J=1.96Hz, 1H), 6.81 (dd, J=2.35Hz, 8.61Hz, 1H), 5.66 (s, 1H), 5.39 (s, 1H), 3.84 (s, 3H), 3.64 (s, 2H), 2.39 (s, 3H).ESI-MS m/z: 219.1 [M+H]+.
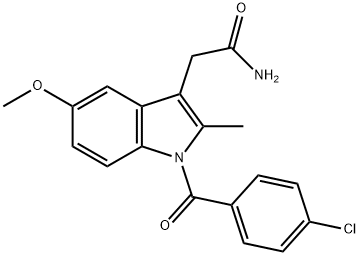
6264-33-1
0 suppliers
inquiry

15992-10-6
28 suppliers
$17.00/100mg
Yield:15992-10-6 99%
Reaction Conditions:
Stage #1: Indomethacin amidewith sodium hydroxide;water in DMF (N,N-dimethyl-formamide) at 20; for 3 h;
Stage #2: with hydrogenchloride in DMF (N,N-dimethyl-formamide);water; pH=9;
Steps:
7
[0257] 5-methoxy-2-methyl-3-indolacetamide (Compound 303). Compound 301 (3.5 g, 9.8 mmol) was dissolved in dry DMF (100 mL) and stirred at room temperature. NaOH (10 N, 20 mL) was slowly added in small quantities over 1 hour while monitoring the reaction by TLC. The reaction was judged complete after 2 hours by TLC. The pH was lowered to 9 by the addition of HCl (4 N). DMF was evaporated via high vacuum rotovap, and syrup was taken up in ethylacetate (600 ml) and washed with sodium bicarbonate (3×300 mL). The aqueous layer was washed with ethylacetate (3×400 ml), and all organic extracts were combined, dried with sodium sulfate, and solvents removed to give 99% product.
References:
US2005/2859,2005,A1 Location in patent:Page/Page column 22

53-86-1
643 suppliers
$18.17/1g

15992-10-6
28 suppliers
$17.00/100mg
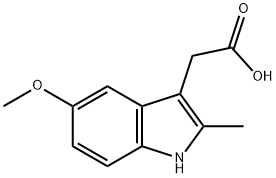
2882-15-7
161 suppliers
$12.00/100mg

15992-10-6
28 suppliers
$17.00/100mg

2882-15-7
161 suppliers
$12.00/100mg
57-13-6
797 suppliers
$5.00/5g

15992-10-6
28 suppliers
$17.00/100mg