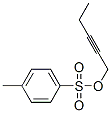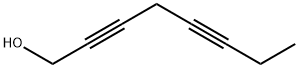
2,5-Octadiyn-1-ol synthesis
- Product Name:2,5-Octadiyn-1-ol
- CAS Number:35378-76-8
- Molecular formula:C8H10O
- Molecular Weight:122.16
Yield:35378-76-8 98%
Reaction Conditions:
with copper(l) iodide;tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
1 Preparation of Compound of Formula 3 (Octa-2, 5-dryn-l-ol:)
To a stirred solution of potassium carbonate (47.8 g, 0.34 mol), Cul (43.9 g, 0.23 mol), and TBAI (85.30 g, 0.23 mol) in DMF (440 mL) cooled to 0 °C, propargyl alcohol (15.52 g, 0.27 mol) was added portion wise at room temperature followed by compound represented by Formula 2 (55 g, 0.23 mol) and the reaction mixture was stirred at room temperature for 16 h. After completion of starting materials, the reaction mixture was cooled to 0 °C and diluted with cold water, ethyl acetate (300 mL x 2), filtered through celite bed and washed with ethyl acetate. The combined organic extracts were washed with cold water (200 mL x 2), brine (100 mL x 2) and dried over anhydrous Na2S04. Solvent was evaporated under reduced pressure to obtain the crude product which was purified by column chromatography (100-200 mesh silica gel, 20 % EtOAc in hexane) to furnish octa-2, 5-diyn-l-ol (55 g, 98 %) as a light red liquid.
References:
WO2013/71411,2013,A1 Location in patent:Page/Page column 18
117606-23-2
0 suppliers
inquiry

35378-76-8
11 suppliers
$120.00/10mg

16400-32-1
151 suppliers
$12.00/250mg

107-19-7
0 suppliers
$12.00/25g

35378-76-8
11 suppliers
$120.00/10mg

13280-07-4
43 suppliers
$60.00/100mg
107-00-6
73 suppliers
$85.00/25g

35378-76-8
11 suppliers
$120.00/10mg