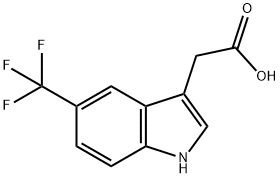
2-(5-Trifluoromethyl-1H-indol-3-yl)acetic acid synthesis
- Product Name:2-(5-Trifluoromethyl-1H-indol-3-yl)acetic acid
- CAS Number:378802-40-5
- Molecular formula:C11H8F3NO2
- Molecular Weight:243.18
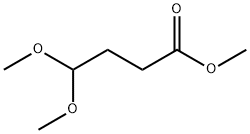
4220-66-0
38 suppliers
$17.00/100mg
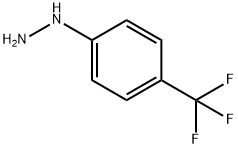
368-90-1
159 suppliers
$15.00/1g

378802-40-5
26 suppliers
$325.00/500mg
Yield:378802-40-5 1.5%
Reaction Conditions:
with sodium hydroxide;sulfuric acid in methanol;water;ethyl acetate;
Steps:
R.77 Synthesis of 2-(5-trifluoromethyl-1H-indol-3-yl)acetic acid
Reference Example 77 Synthesis of 2-(5-trifluoromethyl-1H-indol-3-yl)acetic acid A mixture of 4-trifluoromethylphenylhydrazine (5 g), 4,4-dimethoxybutyric acid methyl ester (6 g) and 10% aqueous sulfuric acid (20 ml) was stirred for 6 hours under a nitrogen atmosphere at 90° C. The reaction mixture was allowed to cool, water was added, and then extraction was performed with ethyl acetate. The organic layer was dried over sodium sulfate and concentrated under reduced pressure. A 2 N sodium hydroxide aqueous solution (10 ml) and methanol (10 ml) were added to the obtained residue, and the mixture was heated to reflux for one hour. Water was added to the reaction mixture which was then rendered acidic with concentrated aqueous hydrochloric acid, extracted with ethyl acetate, dried over sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography using a mixed solvent of dichloromethane, methanol, and acetic acid to obtain 0.11 g of the title compound as a colorless powder (1.5% yield). The NMR data for the obtained compound were as follows. NMR: (300 MHz, DMSO-d6) δ: 3.72 (2H,s), 7.37 (1H,dd), 7.43 (1H,d), 7.54 (1H,d), 7.8-8.0 (1H,m), 11.38 (1H,s), 12.0-12.4 (1H,m)
References:
US2003/162724,2003,A1
100846-24-0
168 suppliers
$35.00/250mg

378802-40-5
26 suppliers
$325.00/500mg