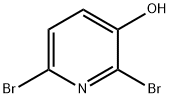
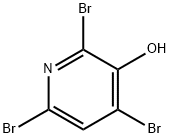
2,6-DIBROMO-3-HYDROXYPYRIDINE synthesis
- Product Name:2,6-DIBROMO-3-HYDROXYPYRIDINE
- CAS Number:6602-33-1
- Molecular formula:C5H3Br2NO
- Molecular Weight:252.89
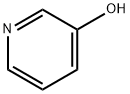
109-00-2

6602-33-1
General procedure for the synthesis of 2,6-dibromo-3-hydroxypyridine from 3-hydroxypyridine: Ice-cold bromine (16.2 mL, 315 mmol) was added dropwise to a stirring 2.5 M sodium hydroxide solution (320 mL, 800 mmol). This mixed solution was then slowly added to a 2.5 M sodium hydroxide solution (110 mL, 275 mmol) containing 3-hydroxypyridine (10.0 g, 105 mmol). The reaction mixture was stirred at 0 °C for 1 hour, followed by continued stirring at room temperature for 4 hours. Upon completion of the reaction, a small amount of precipitate was removed by filtration. The filtrate was cooled and the pH was adjusted to 1 with concentrated hydrochloric acid, at which point a solid precipitated. The solid product was collected by filtration, washed with water, dried, and recrystallized from carbon tetrachloride to give 2,6-dibromo-3-hydroxypyridine (12.1 g, 46% yield) as a beige solid. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ= 11.14 (br s, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H); mass spectra (ES-ve) m/z: 250, 252, 254 (M-H)-.

109-00-2
610 suppliers
$10.00/10g

6602-33-1
115 suppliers
$14.00/250mg
Yield: 46%
Reaction Conditions:
with bromine;sodium hydroxide at 0 - 20; for 5 h;
Steps:
40
5.40
2,6-Dibromopyridin-3-ol (30)
An ice-cold solution of bromine (16.2 mL, 315 mmol) in sodium hydroxide (2.5 M, 320 mL, 800 mmol) was added dropwise to a stirred solution of pyridin-3-ol (10.0 g, 105 mmol) in sodium hydroxide (2.5 M, 110 mL, 275 mmol).
The solution was stirred at 0 °C for 1 h and then at room temp for 4 h.
A small amount of precipitate that formed was removed by filtration.
The filtrate was cooled, and concentrated hydrochloric acid was added until the pH reached 1.
The solid was collected by filtration, washed with water, dried, and recrystallised from carbon tetrachloride to give 30 (12.1 g, 46%) as a beige coloured solid: 1H NMR (400 MHz, DMSO-d6) δ = 11.14 (br s, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H); MS ES-ve m/z 250, 252, 254 (M-H)-.
References:
Miah, Afjal H.;Abas, Hossay;Begg, Malcolm;Marsh, Benjamin J.;O'Flynn, Daniel E.;Ford, Alison J.;Percy, Jonathan M.;Procopiou, Panayiotis A.;Richards, Steve A.;Rumley, Sally-Anne [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 15,p. 4298 - 4311]

109-00-2
610 suppliers
$10.00/10g
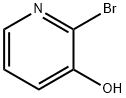
6602-32-0
420 suppliers
$10.00/1g

6602-33-1
115 suppliers
$14.00/250mg

109-00-2
610 suppliers
$10.00/10g

6602-33-1
115 suppliers
$14.00/250mg
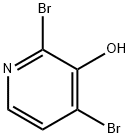
129611-31-0
23 suppliers
inquiry

109-00-2
610 suppliers
$10.00/10g

6602-33-1
115 suppliers
$14.00/250mg
6602-34-2
52 suppliers
$120.00/1g

109-00-2
610 suppliers
$10.00/10g

6602-32-0
420 suppliers
$10.00/1g

6602-33-1
115 suppliers
$14.00/250mg

6602-34-2
52 suppliers
$120.00/1g