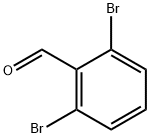
2,6-DIBROMOBENZALDEHYDE synthesis
- Product Name:2,6-DIBROMOBENZALDEHYDE
- CAS Number:67713-23-9
- Molecular formula:C7H4Br2O
- Molecular Weight:263.91
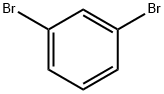
108-36-1

68-12-2

67713-23-9
To a tetrahydrofuran (THF, 180 mL) solution of diisopropylamine (iPr2NH, 21.2 mL, 150 mmol) was slowly added n-butyllithium (n-BuLi, 1.6 M, 94 mL, 150 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for 30 min and then cooled to -78 °C. Subsequently, a solution of 1,3-dibromobenzene (17.6 g, 74.6 mmol) in THF (80 mL) was slowly added over 20 min. The mixture was continued to be stirred at -78 °C for 30 min and then N,N-dimethylformamide (DMF, 11.6 mL, 150 mmol) was added. The reaction mixture was stirred at -78 °C for 1 h. The reaction was quenched with 2.5 M sulfuric acid (H2SO4, 350 mL) and extracted with a solvent mixture of ethyl acetate/ether (50/50, 3 x 300 mL). The organic phases were combined, dried with anhydrous magnesium sulfate (MgSO4), filtered and the filtrate was concentrated to give 2,6-dibromobenzaldehyde (17.6 g, 89% yield). The product was characterized by 1H NMR (250 MHz, CDCl3): δ 10.3 (s, 1H), 7.65 (d, J = 8.0 Hz, 2H), 7.23 (t, J = 8.1 Hz, 1H).
98-11-3
403 suppliers
$19.50/100g

67713-23-9
191 suppliers
$15.00/1g
Yield:-
Steps:
Multi-step reaction with 3 steps
1: N-Bromosuccinimide / chloroform / 2.5 h / 40 - 50 °C
2: n-butyllithium; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 1.17 h / -60 - 0 °C
3: phosphoric acid; hydroxyapatite / 1 h / 150 °C / Inert atmosphere
References:
Henan Normal University;Chen Qi;Wang Jiahao;Zhou Yingjie CN109400453, 2019, A
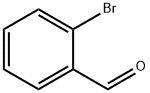
6630-33-7
427 suppliers
$9.00/10g

67713-23-9
191 suppliers
$15.00/1g
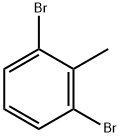
69321-60-4
207 suppliers
$11.00/1g

67713-23-9
191 suppliers
$15.00/1g
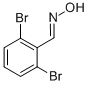
89692-43-3
3 suppliers
inquiry

67713-23-9
191 suppliers
$15.00/1g
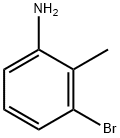
55289-36-6
296 suppliers
$5.00/1g

67713-23-9
191 suppliers
$15.00/1g