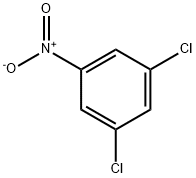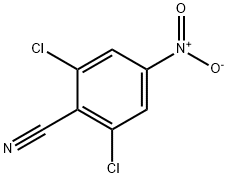
2,6-dichloro-4-nitrobenzonitrile synthesis
- Product Name:2,6-dichloro-4-nitrobenzonitrile
- CAS Number:2112-17-6
- Molecular formula:C7H2Cl2N2O2
- Molecular Weight:217.01
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in water;dimethyl sulfoxide;
Steps:
7.a (a)
(a) 2,6-Dichloro-4-nitrobenzylnitrile (Methylthio)acetonitrile (43.7 cm3) was added to a stirred solution of 1,3-dichloro-5-nitrobenzene (100 g) in dimethylsulphoxide (800 cm3) under nitrogen. Powdered sodium hydroxide (41.7 g) was then added to the mixture in a single portion with initial cooling. After 24 h stirring at room temperature, water (500 cm3) was added to the cooled mixture followed by dilute hydrochloric acid (700 cm3) until pH 1 was attained. The dark mixture was partitioned between diethyl ether-dichloromethane (1:1 by volume, 1 liter) and water (1 liter). The layers were separated and the organic phase further extracted with water (3*500 cm3) then dried and evaporated to a viscous oil. The oil was purified by chromatography over silica gel with an ethyl acetate--petroleum ether gradient to yield a tanned solid, m.p. 102°-4° C. (38 g). 1 Hnmr (90 MHz) δH (CDCl3) 8.30 (S, 2H, Ar--H), 4.10 ppm (S, 2H, CH2) ir spectrum y(KBr) 2250 cm-1 (CN)
References:
US4602041,1986,A
37841-25-1
1 suppliers
inquiry

2112-17-6
4 suppliers
inquiry