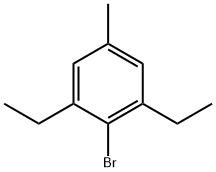
2,6-Diethyl-4-methylbromobenzene synthesis
- Product Name:2,6-Diethyl-4-methylbromobenzene
- CAS Number:314084-61-2
- Molecular formula:C11H15Br
- Molecular Weight:227.14
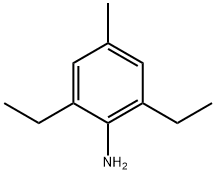
24544-08-9

314084-61-2
General procedure for the synthesis of 2-bromo-1,3-diethyl-5-methylbenzene from 2,6-diethyl-4-methylaniline: 5.2 g (0.4 mol) of 2,6-diethyl-4-methylaniline was dissolved in 280 g of hydrobromic acid (40% by mass) and cooled down to 0°C. Slowly, an aqueous sodium nitrite solution was added dropwise (prepared by dissolving 33.1 g of 0.48 mol sodium nitrite in 100 mL of water). dissolved in 100 mL of water), and after the dropwise addition, stirring was continued for 30 minutes. Subsequently, 26.2 g (0.2 mol) of cuprous bromide was added and the reaction mixture was heated to 60 °C and maintained at this temperature for 4 hours. The progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the mixture was poured into 250 mL of ice water and extracted three times with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and the solvent was evaporated to give 92.36 g of crude product. Fractions with boiling points of 102-106 °C/5 mmHg were collected by distillation under reduced pressure, resulting in 81.07 g of the target product 2-bromo-1,3-diethyl-5-methylbenzene in 89.2% yield.

24544-08-9
184 suppliers
$19.00/250mg

314084-61-2
100 suppliers
$26.00/1g
Yield:314084-61-2 95%
Reaction Conditions:
Stage #1:2,6-diethyl-4-methylaniline with hydrogen bromide at 10; for 0.5 h;
Stage #2: with sodium nitrite at -5 - 0; for 2.5 h;
Stage #3: with ferrous(II) sulfate heptahydrate;hydrogen bromide;sodium bromide in water at 65 - 75; for 0.5 h;Reagent/catalyst;
Steps:
1-4
1 Add 168.5 g (1.0 mol) of 48 wt% hydrobromic acid to a 1 L four-necked flask, cool to 10 ° C, and add 23.0-diethyl-4-methylaniline 163.0 g (1.0 mol) dropwise. Stir for 30min, then cool to -5 ° C, slowly add 25wt% sodium nitrite aqueous solution 303.5g (1.1mol), control the temperature during the addition process -5 ~ 0 ° C, the addition time is about 2h, the reaction is completed for 30min, A diazo solution is obtained.2 Immediately add the diazonium droplet obtained in the step 1 to 27.8 g (0.1 mol) of ferrous sulfate heptahydrate and 10.3 g (0.1 mol) of sodium bromide.48 wt% hydrobromic acid 168.5 g (1.0 mol) and 200 g water in a 2 L four-necked flask,During the dropwise addition process, the temperature is controlled from 65 ° C to 75 ° C, and the reaction is carried out at this temperature for 30 min.Stop the reaction. Add 100 g of dichloromethane to the reacted system and stir for 30 min.The layers were allowed to stand, and the aqueous layer was further extracted with 100 g of dichloromethane, and the organic layers were combined.After evaporating the solvent under reduced pressure, 214.0 g of product was obtained in a yield of 94.3%.The purity was 97.5% (HPLC).
References:
Jiangsu Nongyong Hormone Engineering Technology Research Center Co., Ltd.;Nanjing Gaoheng Biological Technology Co., Ltd.;Zou Peipei;Jiang Xuming;Yang Chuanpeng;Liu Xiaojia;Sun Yonghui CN110105164, 2019, A Location in patent:Paragraph 0022-0028

24544-08-9
184 suppliers
$19.00/250mg
35050-88-5
6 suppliers
inquiry

314084-61-2
100 suppliers
$26.00/1g