2,6-HEPTANEDIONE synthesis
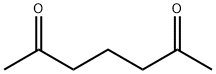
- Product Name:2,6-HEPTANEDIONE
- CAS Number:13505-34-5
- Molecular formula:C7H12O2
- Molecular Weight:128.17
Yield:13505-34-5 40%
Reaction Conditions:
in tetrahydrofuran at 0 - 20; for 2.5 h;
Steps:
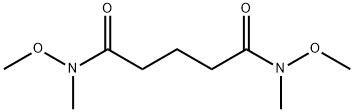
5.5; 7.2 [0890] Step 5: Synthesis of heptane-2,6-dione.
[0891] Methyl magnesium bromide (250 mL, 0.756 mol) was added to a stirred solution of N1,N5-dimemoxy-N1,N5-dimetliylglutaramide (55 g, 0.252 mol) in 800 mL of dry THF at 0°C over 30 min. After all the Grignard reagent was added, the reaction mixture was allowed to warm to room temperature and stirred for 2 h. Water, 50 mL, was added to the mixture followed by 1 % HCl aqueous solution 300 mL. The mixture was extracted with ethyl acetate (1000 mL X 3). The combined organic layers were washed with 1 % HCl aqueous solution, brine, dried over anhydrous Na2S04, concentrated under reduced pressure and purified by silica gel column (10-50% ethyl acetate in petroleum ether) to afford 13 g of heptane-2,6-dione as brownish solid (40% yield). LCMS: m/z 129.1 [M+H]+; = 1.24 min.
References:
SKYHAWK THERAPEUTICS, INC.;LUZZIO, Michael;MCCARTHY, Kathleen;HANEY, William WO2019/28440, 2019, A1 Location in patent:Paragraph 0890-0891; 0934-0935
259236-21-0
3 suppliers
inquiry

13505-34-5
27 suppliers
inquiry

3070-53-9
87 suppliers
$96.00/1mL

13505-34-5
27 suppliers
inquiry

2396-63-6
113 suppliers
$24.00/1mL

13505-34-5
27 suppliers
inquiry