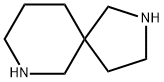
2,7-diazaspiro[4.5]decane synthesis
- Product Name:2,7-diazaspiro[4.5]decane
- CAS Number:40865-50-7
- Molecular formula:C8H16N2
- Molecular Weight:140.23
![2,7-Diazaspiro[4.5]decane-1,3,6,8-tetrone](/CAS/20210305/GIF/1433194-74-1.gif)
1433194-74-1
0 suppliers
inquiry
![2,7-diazaspiro[4.5]decane](/CAS2/GIF/40865-50-7.gif)
40865-50-7
22 suppliers
inquiry
Yield:40865-50-7 48%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 48 h;Inert atmosphere;Reflux;
Steps:
32
4.32
2,7-Diazaspiro[4.5]decane (7)
Bisimide 33 (1.7 g; 8.67 mmol) was suspended in dry THF (30 mL) in 0-5 °C under N2 and 1 M solution of LiAlH4 in THF (40 mL; 40 mmol) was added dropwise.
The ice bath was removed and the mixture was heated in reflux for 2 days under N2.
It was cooled to 0-5 °C, diluted with THF (10 mL) and the following were slowly added dropwise in 0-5 °C: water (1.8 mL), 15% NaOH aq (1.8 mL) and water (5.5 mL).
The resulting slurry was filtered through Celite, the solids were washed with THF (250 mL) and the combined filtrates were concentrated in vacuo to give a yellow oil, which was distilled under vacuum (bp 70 °C at 1.0 mmHg) to give the product 7 (580 mg; 48%) as a colourless oil. δH (400 MHz, CD3OD) 2.88, 2.86 (2H, 2d, J 7.1, CH2NH five-membered), 2.74 (1H, d, J 11.4, CH2NH), 2.74-2.65 (2H, m, CH2CH2NH), 2.65-2.58 (2H, m, CH2CH2CH2NH), 2.57 (1H, d, J 11.4, CH2NH six-membered), 1.72-1.45 (6H, m, CH2CH2CH2NH, CH2CH2CH2NH, CH2CH2NH); δC (100.5 MHz, CD3OD) 56.1 (), 54.6 (CH2NH), 45.6 (CH2CH2CH2NH), 45.2 (CH2CH2NH), 42.5 (C 4°), 36.8 (CH2CH2NH), 35.1 (CH2CH2CH2NH), 23.8 (CH2CH2CH2NH); m/z (CI) 141 (M+H+); HRMS: found: M+H+, 141.1385. C8H17N2 requires 141.1392.
References:
Weinberg, Kamil;Stoit, Axel;Kruse, Chris G.;Haddow, Mairi F.;Gallagher, Timothy [Tetrahedron,2013,vol. 69,# 23,p. 4694 - 4707]

224637-77-8
0 suppliers
inquiry
![2,7-diazaspiro[4.5]decane](/CAS2/GIF/40865-50-7.gif)
40865-50-7
22 suppliers
inquiry