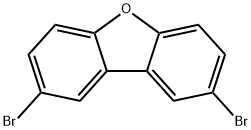
2,8-DIBROMODIBENZOFURAN synthesis
- Product Name:2,8-DIBROMODIBENZOFURAN
- CAS Number:10016-52-1
- Molecular formula:C12H6Br2O
- Molecular Weight:325.98
132-64-9

10016-52-1
General procedure for the synthesis of 2,8-dibromodibenzofuran from dibenzofuran: 1a) Synthesis of 2,8-dibromodibenzofuran: A solution of bromine (92.6 g, 0.58 mol) in acetic acid (54 g) was added slowly and dropwise at 75 °C to a solution of dibenzofuran (23.2 g, 0.14 mol) in acetic acid (232 g). The reaction mixture was stirred continuously at 75°C for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and slowly poured into deionized water. The precipitated orange solid was washed sequentially with aqueous sodium thiosulfate and deionized water. The crude product was purified by recrystallization from n-hexane to give a white solid product in 38% yield with a melting point of 226 °C. The structure of the product was confirmed by 1H-NMR (CDCl3, ppm): 7.65 (d, 2H), 7.59 (dd, 2H), 8.03 (d, 2H).

132-64-9
364 suppliers
$5.00/10g

10016-52-1
165 suppliers
$8.00/250mg
Yield:10016-52-1 87%
Reaction Conditions:
with bromine in acetic acid at 120; for 6 h;Inert atmosphere;
Steps:
4.3. Synthesis and characterizations of L2
To a round bottom flask, Diphenylene-oxide (8.40 g, 50 mmol), bromine (2.6 mL) dissolved in 30 mL glacial acetic acid were added. The resultant suspension was heating for 6 h under nitrogen at 120 C. After cooling to 25 C, the intermediate product 2 was recovered by filtration and recrystallization in acetic acid and vacuum drying as a white solid (12.2 g, 75%). 1H NMR (400 MHz,CDCl3) d (ppm): 8.03 (s, 2H), 7.58 (d, J 8.0 Hz, 2H), 7.44 (d, J 8 Hz,2H) (Fig. S27) [46]. To a solution of 2 (0.324 g, 1.0 mmol) and 4-([2,2': 6', 2''- terpyridyl]-4'-) - benzene boric acid (0.88 g,2.50 mmol) in THF (100 mL), aqueous NaOH (160 mg, 4.0 mmol) was added. The mixture was degassed for 10 min, then Pd(PPh3)4(115 mg, 0.10 mmol) was added. Following the procedure L1, thepure product L2was obtained as a white solid (0.55 g, 70%).
References:
Liu, Qianqian;Yang, Xiaoyu;Wang, Meng;Liu, Die;Chen, Mingzhao;Wu, Tun;Jiang, Zhiyuan;Wang, Pingshan [Tetrahedron,2019,vol. 75,# 16,p. 2400 - 2405] Location in patent:supporting information

132-64-9
364 suppliers
$5.00/10g

10016-52-1
165 suppliers
$8.00/250mg
86-76-0
218 suppliers
$6.00/1g

132-64-9
364 suppliers
$5.00/10g

10016-52-1
165 suppliers
$8.00/250mg
67733-57-7
0 suppliers
inquiry
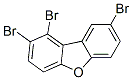
84761-81-9
1 suppliers
inquiry
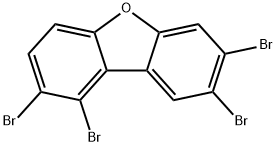
84761-80-8
2 suppliers
inquiry
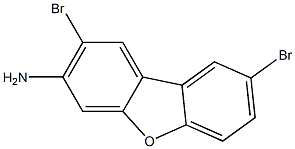
92059-21-7
0 suppliers
inquiry

10016-52-1
165 suppliers
$8.00/250mg
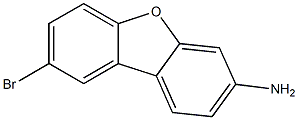
106104-18-1
0 suppliers
inquiry

10016-52-1
165 suppliers
$8.00/250mg