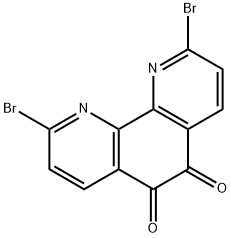
2,9-dibromo-1,10-phenanthroline-5,6-dione synthesis
- Product Name:2,9-dibromo-1,10-phenanthroline-5,6-dione
- CAS Number:943861-95-8
- Molecular formula:C12H4Br2N2O2
- Molecular Weight:367.98
39069-02-8
137 suppliers
$15.00/100mg

943861-95-8
41 suppliers
inquiry
Yield:943861-95-8 73%
Reaction Conditions:
Stage #1:2,9-dibromo-1,10-phenanthroline with sulfuric acid;nitric acid;potassium bromide at 0 - 80; for 3 h;
Stage #2: with sodium hydroxide in water; pH=7Cooling with ice;Temperature;
Steps:
4.3.1 General procedure I: oxidation of bromo-phens
General procedure: Bromo-phen (4-bromo-phen, 3-bromo-phen, 2-bromo-phen, 3,8-dibromo-phen, 2,9-dibromo-phen, 3.86mmol) and KBr (4.59g, 38.6mmol) were added into a mixture of HNO3 (1.4g/mL, 10mL) and H2SO4 (1.84g/mL, 20mL) at 0°C, and the mixture was heated under stirring (80-110°C for 3h). After cooled to room temperature, the solution was poured into a mixture of ice (100g) and water (200mL) and neutralized carefully with a 1mol/L sodium hydroxide solution until the pH=7. The suspension was filtrated, washed with water and dried in vacuo. Purification by silica gel column chromatography using CHCl3 as the eluent gave corresponding bromo-phds (1a-1e) with yields of 11-94%, as shown in Scheme 1. It is worth pointing out that another two dibromo-phds 2a and 2c could be also isolated in the temperature of 90-110°C with 10-55% yields during the syntheses of 1a and 1c. In addition, two hydroxylation products, i.e. 3 and 5, were separated successfully in the after-treatment process of 4-bromo-phen oxidative reaction.
References:
Peng, Yu-Xin;Hu, Bin;Huang, Wei [Tetrahedron,2018,vol. 74,# 34,p. 4495 - 4503]
66-71-7
406 suppliers
$6.00/5g

943861-95-8
41 suppliers
inquiry