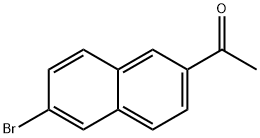
2-Acetyl-6-bromonaphthalene synthesis
- Product Name:2-Acetyl-6-bromonaphthalene
- CAS Number:1590-25-6
- Molecular formula:C12H9BrO
- Molecular Weight:249.1
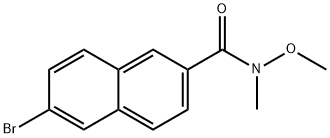
861880-64-0

75-16-1

1590-25-6
Under nitrogen protection, 6-bromo-N-methoxy-N-methyl-2-naphthalenecarboxamide (82.9 g, 282 mmol, 1.0 eq.) was dissolved in tetrahydrofuran (600 mL) in a four-necked flask. The reaction mixture was cooled in an ice bath at a controlled temperature in the range of 10-15 °C. Methylmagnesium bromide (3.2 M in methyltetrahydrofuran, 197 mL, 2.2 eq.) was added slowly dropwise over a period of 1 hour. After the dropwise addition was completed, the reaction mixture continued to be stirred in an ice bath for 30 minutes. Subsequently, aqueous 2M hydrochloric acid (100 mL) was carefully added dropwise to quench the reaction under cooling in the ice bath. After the organic solvent was removed by evaporation, the precipitated product was extracted with dichloromethane (500 mL). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated. The resulting solid residue was dried under vacuum at 40 °C to give 1-(6-bromonaphthalen-2-yl)ethanone (70.6 g, 99% yield).

861880-64-0
8 suppliers
inquiry

1590-25-6
77 suppliers
$10.00/100mg
Yield: 99%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;2-methyltetrahydrofuran;methylmagnesium bromide
Steps:
2.3 Step 3
Step 3 6-bromo-N-methoxy-N-methyl-2-naphthamide (82.9 g, 282 mmol, 1 equiv.) was dissolved in tetrahydrofurane (600 mL) in a 4-neck flask under nitrogen. The reaction mixture was cooled in an ice bath and methyl magnesium bromide (3.2 M in methyl-tetrahydrofurane, 197 mL, 2.2 equiv.) was added drop wise during 1 hour, while maintaining the temperature of the reaction mixture between 10-15° C. The reaction mixture was stirred 30 minutes further in an ice bath. Aqueous hydrochloric acid (2 M, 100 mL) was then carefully added drop wise while cooling on an ice bath. The organic solvent was evaporated and the precipitated product extracted with dichloromethane (500 mL). The solution was dried over sodium sulphate, filtered and concentrated. The solid residue was dried in vacuum at 40° C. yielding 1-(6-bromonaphthalen-2-yl)-ethanone (70.6 g, 99%). Alternative for Preparation of 1-(6-bromonaphthalen-2-yl)ethanone
References:
Vandyck, Koen;Last, Stefaan Julien;Houpis, Ioannis Nicolaos;Raboisson, Pierre Jean-Marie Bernard US2012/219594, 2012, A1

861880-64-0
8 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml

1590-25-6
77 suppliers
$10.00/100mg
580-13-2
506 suppliers
$6.00/1g

75-36-5
588 suppliers
$17.92/100G

1590-25-6
77 suppliers
$10.00/100mg
201230-82-2
1 suppliers
inquiry
594-27-4
121 suppliers
$28.00/5g
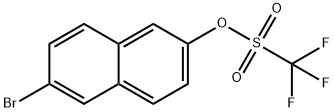
151600-02-1
58 suppliers
$23.00/250mg

1590-25-6
77 suppliers
$10.00/100mg

861880-64-0
8 suppliers
inquiry
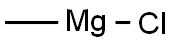
676-58-4
292 suppliers
$15.00/25ml

1590-25-6
77 suppliers
$10.00/100mg