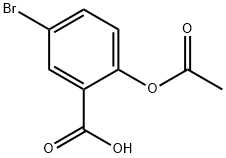
2-Acetyloxy-5-bromobenzoic acid synthesis
- Product Name:2-Acetyloxy-5-bromobenzoic acid
- CAS Number:1503-53-3
- Molecular formula:C9H7BrO4
- Molecular Weight:259.05
Yield:1503-53-3 98%
Reaction Conditions:
with pyridine in dichloromethane;
Steps:
100 Example 100; [(2S)-[5-BROMO-2-(4-TRIFLUOROMETHYLBENZYLOXY)-BENZOYLAMINE]-3-(2'-PHENOXYBIPHENYL-4-YL)-]propionic acid
5-Bromo-salicylic acid (2.16 g, 10 [MMOL)] was first transformed into [2-ACETYL-5-BROMO-] salicylic acid (252 g, 98%) with acetyl chloride (2.34 g, 30 mmol) and pyridine (3.95 g, 50 [MMOL)] in DCM. The above acid (1.29 g, 5.0 [MMOL)] was converted into acid chloride by using oxyl chloride (1.97 g, 15 [MMOL)] and catalytic amount of DMF in DCM, then 2-phenoxy- biphenyl alanine (1.45 g, 5.0 [MMOL)] and DIEA (0.77 g, 6.0 [MMOL)] were added to the acid chloride to form [(2SUS] [[5-BROMO-2-HYDROXYBENZOYLAMINE]-3- (2'-PHENOXYBIPHENYL-4-YL)-] propionic acid methyl ester (1.92 g, 85%). The above methyl ester (50 mg, 0.092 [MMOL)] was reacted with 4-trifluoromethyl benzyl bromide (44 mg, 0.18 [MMOL)] as described in general procedure H to provide [(2S)- [5-BROMO-2- (4-TRIFLUOROMETHYLBENZYLOXY}-BENZOYLAMINE]-3- (2'-] phenoxybiphenyl-4-yl)-propionic acid methyl ester (55 mg, 85%). The ester was [HYDROLYZED] following general procedure C to give the title compound (52 mg, 96%). 'H NMR (400 MHz, [CDC13)] : 3.03, 3.22 (ABX, 2H), 4,92 (m, 3H), 6.64 (d, [1H),] 6.76 (m, 2H), 6.85 (dd, 1 H), 6.93 (m, 2H), 7.00 (d, 2H), 7.07-7. 24 (m, 7H), 7.39 (m, 4H), 8.22 (d, 1 H), 8.26 (d, [1H)] ; LC/MS (m/z) : 690 (M+1) +.
References:
WO2004/14844,2004,A2 Location in patent:Page 203
89-55-4
266 suppliers
$9.00/5g

1503-53-3
24 suppliers
inquiry

