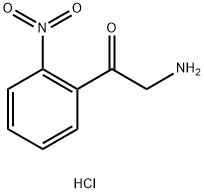
2-AMINO-1-(2-NITRO-PHENYL)-ETHANONE HYDROCHLORIDE synthesis
- Product Name:2-AMINO-1-(2-NITRO-PHENYL)-ETHANONE HYDROCHLORIDE
- CAS Number:23082-65-7
- Molecular formula:C8H9ClN2O3
- Molecular Weight:216.62
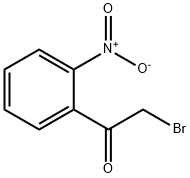
6851-99-6
161 suppliers
$12.00/1g

23082-65-7
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;hexamethylenetetramine in sodium hydroxide;ethanol;dichloromethane;
Steps:
26 5-o-Nitrophenyl-2-(1-normon-2-yl)oxazole A
o-Nitrophenacylbromide (9.45 g, 38 mmol) was dissolved in dichloromethane (100 ml) and hexamethylenetetramine (5.4 g, 38 mmol) added and the mixture stirred for 1 h. The reaction was then filtered to yield a pale green solid m.p. 136°-138° C. (7.38 g, 50%). This solid was added to ethanol (40 ml) and concentrated hydrochloric acid (10 ml) and stirred for 3 h then left standing for 2 days. The reaction mixture was filtered, and the residue was washed with water, and dried to yield a pale yellow solid. This was dissolved in aqueous sodium hydroxide, extracted with ethyl acetate, and the extracts evaporated under reduced pressure. Dilute hydrochloric acid was added to the residue and the resulting solution filtered and the filtrate evaporated under reduced pressure to yield o-nitrophenacylammonium chloride as a dark brown solid (1.45 g, 39%); δH ((CD3)2 SO) 8.8 (1H, S, NH), 8.4-7.8 (4H, m, aryl), 4.6 (S, CH2).
References:
US4812470,1989,A