
2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE synthesis
- Product Name:2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE
- CAS Number:23474-66-0
- Molecular formula:C13H10N2O2
- Molecular Weight:226.23
![2-Nitrodibenzo[b,f][1,4]oxazepin-11(10H)-one](/CAS/GIF/16398-16-6.gif)
16398-16-6
5 suppliers
inquiry
![2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE](/StructureFile/ChemBookStructure3/GIF/CB9206720.gif)
23474-66-0
15 suppliers
$178.00/100mg
Yield:23474-66-0 94%
Reaction Conditions:
with iron(III) chloride;iron in ethanol;water; for 4 h;Heating / reflux;
Steps:
12.3
Step 3: 2-nitrobenzo[b,f][1,4]oxazepin-11(10H)-one (2.00 g, 7.81 mmols) was suspended in water and abs. ethanol (25 mls+25 mls) and the suspension treated with elemental iron (0.36 g, 6.42 mmols) and iron (III) chloride (65 mg, 0.4 mmols). The suspension was refluxed for a total of 2.5 hrs. A further portion of iron (0.33 g) was added at 30 minutes and then again at 1 hour to the refluxing mixture. The mixture was then poured into excess ethanol and filtered off from the iron residues. The filtrate was stripped of ethanol under reduced pressure and the residue taken up in an excess volume of water. The product was filtered off and dried by suction. This gave 1.66 g (94% yield) of the amine as a light brick coloured solid.HPLC (A)=2.19'; MS[Ices+] MH+=227.2
References:
US2008/275023,2008,A1 Location in patent:Page/Page column 6
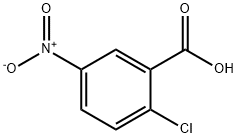
2516-96-3
453 suppliers
$6.00/25g
![2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE](/StructureFile/ChemBookStructure3/GIF/CB9206720.gif)
23474-66-0
15 suppliers
$178.00/100mg
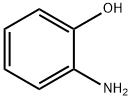
95-55-6
556 suppliers
$5.00/5G
![2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE](/StructureFile/ChemBookStructure3/GIF/CB9206720.gif)
23474-66-0
15 suppliers
$178.00/100mg
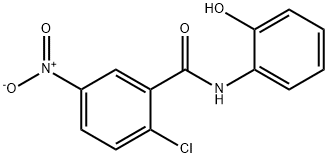
16398-07-5
6 suppliers
inquiry
![2-AMINO-10H-DIBENZO[B,F][1,4]OXAZEPIN-11-ONE](/StructureFile/ChemBookStructure3/GIF/CB9206720.gif)
23474-66-0
15 suppliers
$178.00/100mg