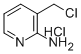
2-AMINO-3-CHLOROMETHYL PYRIDINE HYDROCHLORIDE synthesis
- Product Name:2-AMINO-3-CHLOROMETHYL PYRIDINE HYDROCHLORIDE
- CAS Number:858431-27-3
- Molecular formula:C6H8Cl2N2
- Molecular Weight:0
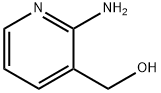
23612-57-9
218 suppliers
$8.00/250mg

858431-27-3
20 suppliers
$45.00/10mg
Yield:858431-27-3 92%
Reaction Conditions:
with thionyl chloride in tetrahydrofuran at 20; for 0.5 h;
Steps:
1.b
Thionyl chloride (12.4mL, 170mmol) was added to a 1L round bottom flask containing tetrahydrofuran (500mL). The flask was cooled in an ice-water bath and a solution of (2- aminopyridin-3-yl) methanol (17. 6g, 142mmol) in tetrahydrofuran (150mL) was added dropwise at a rate such that the temperature remained below 10°C. The reaction mixture was warmed to room temperature and stirred for an additional 30 minutes. The solvent was evaporated and the residual solid was ground to a fine powder with a mortar and pestle, then dried under vacuum to give 23.2g (92%) of the title compound as a yellow powder.'H NMR (CDC13) 8 4.63 (s, 2H), 6.87 (m, 1H), 7.58 (br s, 2H), 7.85 (m, 2H).
References:
WO2005/95391,2005,A1 Location in patent:Page/Page column 32
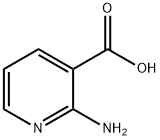
5345-47-1
393 suppliers
$9.00/1g

858431-27-3
20 suppliers
$45.00/10mg