
2-AMINO-5-BROMO-3-FLUOROBENZOIC ACID synthesis
- Product Name:2-AMINO-5-BROMO-3-FLUOROBENZOIC ACID
- CAS Number:874784-14-2
- Molecular formula:C7H5BrFNO2
- Molecular Weight:234.02
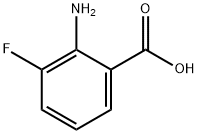
825-22-9

874784-14-2
Step 1: 2-Amino-3-fluorobenzoic acid (1.0 g, 6.45 mmol) was dissolved in N,N-dimethylformamide (DMF, 10 mL) at -10 °C. Subsequently, a solution of N-bromosuccinimide (NBS, 1.15 g, 6.45 mmol) in DMF (4 mL) was added dropwise to the above solution over 10 min. After the dropwise addition was completed, the reaction mixture was kept at -10°C and stirring was continued for 1 hour. Upon completion of the reaction, the reaction was quenched with aqueous sodium bisulfate (50 mL), at which point a large amount of solid precipitated. The solid product was collected by filtration and dried under vacuum to give 2-amino-5-bromo-3-fluorobenzoic acid (1.2 g, yield: 80%) as a yellow solid.

825-22-9
347 suppliers
$13.00/5g

874784-14-2
93 suppliers
$16.00/250mg
Yield:874784-14-2 82%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 0 - 20; for 1 h;
Steps:
1.1 Step 1 : 2-amino-5-bromo-3-fluoro-benzoic acid
2-Amino-3-fluoro-benzoic acid (2.00 g, 12.9 mmol) was suspended in dichloromethane (34.0 mL) and N-bromosuccinimide (2.39 g, 12.9 mmol) was added in small portions at 0 °C. The reaction mixture was stirred at room temperature for 1 hour. The reaction mixture was filtered and the solid was washed with dichloromethane (30 mL) and dried in air for 15 min to give the desired product as a white solid (2.46 g, 10.5 mmol, 82% Yield). LCMS: 0.82 min; ES+ 234/236 ( M+H+); XH NMR (DMSO-d6, 400MHz): δ (ppm) 7.62-7.66 (s, 1H), 7.52 (d, 1H).
References:
WO2018/17490,2018,A1 Location in patent:Paragraph 0153; 0154