
2-AMINO-5-BROMO-3-METHYLBENZOIC ACID synthesis
- Product Name:2-AMINO-5-BROMO-3-METHYLBENZOIC ACID
- CAS Number:206548-13-2
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
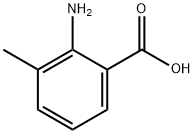
4389-45-1

206548-13-2
Step A: 2-Amino-3-methylbenzoic acid (10.0 g, 66 mmol) was dissolved in DMSO, followed by the addition of 48% aqueous hydrogen bromide solution (18.0 mL). The reaction mixture was stirred at room temperature overnight, and upon completion of the reaction, the reaction was quenched with saturated sodium bicarbonate solution. The resulting mixture was continued to be stirred overnight and the precipitate was subsequently collected by filtration and dried under vacuum to afford 2-amino-5-bromo-3-methylbenzoic acid (13.0 g, 85% yield) as a pink solid. The product was characterized by 1H NMR (500 MHz, DMSO): δ 7.69 (d, J = 1.0 Hz, 1H), 7.33 (d, J = 1.0 Hz, 1H), 2.10 (s, 3H); mass spectrum (ESI+) m/z 231 ([M + H]+).

4389-45-1
505 suppliers
$5.00/5g

206548-13-2
107 suppliers
$11.00/250mg
Yield: 100%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
17.1 Step 1 :
To a solution of 2-amino-3-methylbenzoic acid (500 mg, 3.31 mmol) in DMF (33 ml.) was added N-bromosuccinimide (618 mg, 3.47 mmol). The reaction mixture was stirred at room temperature for 1 h before being poured in water (50 ml.) and extracted with ethyl acetate (3 x 50 ml_). The combined organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under vacuum to give 2-amino-5- bromo-3-methyl-benzoic acid (760 mg, quantitative yield) as a brown solid. (1109) M/Z (M[79Br]+H)+ = 230.0.
References:
DOMAIN THERAPEUTICS;AMALRIC, Camille;BARRE, Anaïs;SCHANN, Stephan;MAYER, Stanislas;DORANGE, Ismet;MANTEAU, Baptiste;BLAYO, Anne-Laure WO2020/21064, 2020, A1 Location in patent:Page/Page column 147
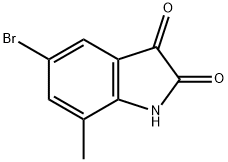
77395-10-9
86 suppliers
$60.00/5 g

206548-13-2
107 suppliers
$11.00/250mg
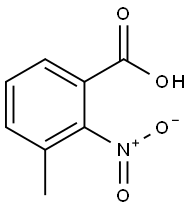
5437-38-7
489 suppliers
$5.00/10g

206548-13-2
107 suppliers
$11.00/250mg
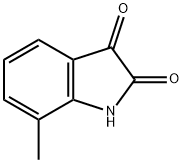
1127-59-9
170 suppliers
$6.00/250mg

206548-13-2
107 suppliers
$11.00/250mg
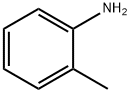
95-53-4
461 suppliers
$10.00/1g

206548-13-2
107 suppliers
$11.00/250mg