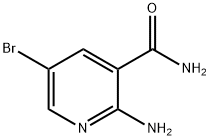
2-AMINO-5-BROMONICOTINAMIDE synthesis
- Product Name:2-AMINO-5-BROMONICOTINAMIDE
- CAS Number:58483-98-0
- Molecular formula:C6H6BrN3O
- Molecular Weight:216.04
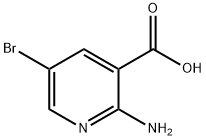
52833-94-0

58483-98-0
2-Amino-5-bromonicotinic acid (2.0 g, 9.20 mmol) was used as raw material, which was mixed with HOBT (1.40 g, 11.2 mmol), EDCI (3.52 g, 18.4 mmol), Et3N (4.68 g, 46.0 mmol) and NH4Cl (2.48 g, 46.0 mmol) in DMF (100 mL) in DMF (100 mL). The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was concentrated under vacuum and subsequently suspended in water and extracted with CH2Cl2. The organic layer was washed with brine, dried over Na2SO4 and concentrated to afford the crude product 2-amino-5-bromonicotinamide (1.80 g, yield: 80%), which could be used in subsequent steps without further purification. The structure of the product was confirmed by 1H NMR (DMSO-d6, 400 MHz) δ 8.14 (dd, J = 4.4 Hz, 2.4 Hz, 2H), 8.04 (s, 1H), 7.46 (s, 1H), 7.37 (s, 2H) and mass spectrum (M + H: 216/218).

52833-94-0
218 suppliers
$13.00/1g

58483-98-0
44 suppliers
$48.00/100mg
Yield:58483-98-0 80%
Reaction Conditions:
with ammonium chloride;benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 25;
Steps:
15.1 Synthesis of2-amino-5-bromonicotinamide
A mixture of 2-amino-5-bromonicotinic acid (2.0 g, 9.20 mmol), HOBT (1.40 g, 11.2 mmol), EDCI (3.52 g, 18.4 mmol), Et3N (4.68 g, 46.0 mmol) and NH4C1 (2.48 g, 46.0 mmol) in DMF (100 mL) was stirred overnight at room temperature. The reaction mixture was concentratedin vacuo, suspended in water and extracted with CH2C12. The organic layer was washed with brine,dried over Na2504 and concentrated to give the product of 2-amino-5-bromonicotinamide (1.80 g,yield: 80%), which was used for the next step without further purification. ‘H NMR (DMSO-d6,400 MHz) 8.14 (dd, J= 4.4 Hz, 2.4 Hz, 2H), 8.04 (s, 1H), 7.46 (s, 1H), 7.37 (s, 2H). MS (M+H):216/218.
References:
WO2014/205593,2014,A1 Location in patent:Page/Page column 55
52963-33-4
22 suppliers
$116.00/500mg

58483-98-0
44 suppliers
$48.00/100mg
5345-47-1
392 suppliers
$9.00/1g

58483-98-0
44 suppliers
$48.00/100mg