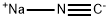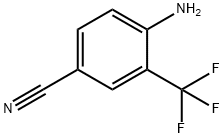
2-Amino-5-cyanobenzotrifluoride synthesis
- Product Name:2-Amino-5-cyanobenzotrifluoride
- CAS Number:327-74-2
- Molecular formula:C8H5F3N2
- Molecular Weight:186.13
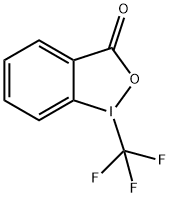
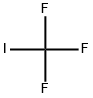
887144-94-7
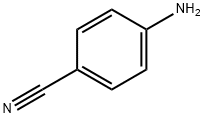
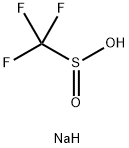
873-74-5

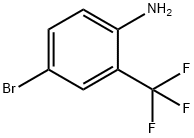
327-74-2
The general steps for synthesizing 2-trifluoromethyl-4-cyanobenzenamine from 1-(trifluoromethyl)-1,2-phenyliodono-3(1H)-one and p-aminobenzonitrile are as follows: in this embodiment of the method of preparing trifluoromethyl aromatic amine, p-cyanobenzenamine was selected as the aromatic amine, and the reaction time was 12 hours, and the rest of the reaction conditions and the post-processing steps were kept the same as in Example 28. The specific reaction process parameters were as follows: 1-(trifluoromethyl)-1,2-phenyliodono-3(1H)-one (0.5 mmol, 1.0 eq.), p-aminobenzonitrile (1.5 mmol, 3.0 eq.), nickel hydroxide (10 mol%), and potassium carbonate (1.5 mmol, 3.0 eq.) were dissolved in DMSO (2 mL), and the reaction was carried out for 2 hr at 35 °C. The subsequent reaction and post-treatment procedures were carried out with reference to Example 1.

887144-94-7
178 suppliers
$19.00/1g

873-74-5
657 suppliers
$9.00/1g

327-74-2
216 suppliers
$14.00/5g
Yield:327-74-2 81%
Reaction Conditions:
with potassium carbonate in acetonitrile at 75; for 6 h;Schlenk technique;Inert atmosphere;
Steps:
3. General procedure for the reaction
General procedure: Experimental Procedure: A dried 25 mL Schlenk tube equipped with a magnetic stir bar was charged with Togni’s Reagent 2 (0.25 mmol, 1.0 equiv), free anilines 1 (0.75 mmol, 3.0 equiv), K2CO3 (0.375 mmol, 1.5 equiv) and CH3CN (1.5 mL). The reaction mixture was then stirred at 75 °C for 6 h under an argon atmosphere. The reaction progress was monitored by TLC. After cooling to room temperature, the mixture was washed with water and extracted with CH2Cl2 three times, then washed with saturated NaCl solution. The combined organic layer was dried with anhydrous Na2SO4 and filtered. The filtrate was concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (Elutent: petroleum ether-EtOAc) to give the pure product. The products were characterized by 1H NMR, 13C NMR, 19F NMR, GC -MS.
References:
Chen, Xiaoyu;Ding, Licheng;Li, Linlin;Li, Jingya;Zou, Dapeng;Wu, Yangjie;Wu, Yusheng [Tetrahedron Letters,2020,vol. 61,# 9,art. no. 151538] Location in patent:supporting information
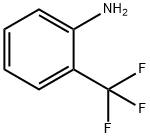
88-17-5
373 suppliers
$14.00/25g

327-74-2
216 suppliers
$14.00/5g