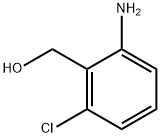
(2-AMINO-6-CHLORO-PHENYL)-METHANOL synthesis
- Product Name:(2-AMINO-6-CHLORO-PHENYL)-METHANOL
- CAS Number:39885-08-0
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
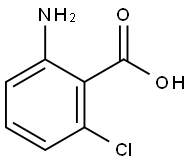
2148-56-3

39885-08-0
General procedure for the synthesis of (2-amino-6-chlorophenyl)-methanol from 2-amino-6-chlorobenzoic acid: 50.0 g (286 mmol) of 2-amino-6-chlorobenzoic acid was dissolved in 300 mL of anhydrous tetrahydrofuran (THF). The resulting solution was cooled to 0-10°C, followed by the slow addition of 450 mL of 1.0 M lithium aluminum hydride in THF solution. The reaction mixture was gradually warmed to room temperature and stirred continuously at room temperature overnight. Upon completion of the reaction, 300 mL of 10% aqueous sodium hydroxide solution was carefully added to the mixture to quench the reaction. The organic phase was separated and washed sequentially with ethyl acetate and water. The organic phase was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent, yielding 42 g (270 mmol, 95% yield) of a yellow solid product, which could be used without further purification.

2148-56-3
370 suppliers
$5.00/10g

39885-08-0
87 suppliers
$6.00/100mg
Yield: 95%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Steps:
3.2 2) Synthesis of (2-amino-6-chlorophenyl)methanol
Dissolves 50.0 g (286 mmol) of 2-amino-6chlorophenyl acid in 300 ml of anhydrous THF (Tetrahydrofuran)(2-Amino-6-chlorobenzoic acid) mixture, cooling the solution to 0 ~ 10 °C,Add 450ml of 1.0M lithium aluminum hydride THF solution,The reaction mixture was allowed to warm to room temperature and then stirred at room temperature overnight.300 ml of 10% NaOH aqueous solution was added to the reaction mixture,The organic phase was separated and washed with ethyl acetate and water. After drying with magnesium sulfate,The solvent was removed in vacuo to give 42 g (270 mmol, 95%) of the product as a yellow solid.No further purification.
References:
Jiguang Technology Co., Ltd.;Yan Fengwen;Zhang Zhenghao CN107722059, 2018, A Location in patent:Paragraph 0094; 0097; 0098; 0099
6575-11-7
134 suppliers
$8.00/1g

39885-08-0
87 suppliers
$6.00/100mg