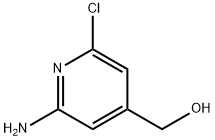
(2-aMino-6-chloropyridin-4-yl)Methanol synthesis
- Product Name:(2-aMino-6-chloropyridin-4-yl)Methanol
- CAS Number:1334294-36-8
- Molecular formula:C6H7ClN2O
- Molecular Weight:158.59
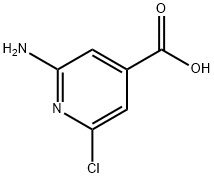
6313-55-9
33 suppliers
$60.00/100mg

1334294-36-8
11 suppliers
inquiry
Yield:1334294-36-8 88%
Reaction Conditions:
Stage #1: 2-amino-6-chloropyridine-4-carboxylic acidwith lithium aluminium tetrahydride in tetrahydrofuran at 20;Inert atmosphere;
Stage #2: with water;potassium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
Intermediate 41 :(2-amino-6-chloro-4-pyridinyl)methanolUnder an atmosphere of nitrogen and at ambient temperature, a solution of lithium aluminium hydride (98.2g, 1.25mol) in tetrahydrofuran (2200ml_) was treated portionwise with 2-amino-6-chloro-4-pyridine carboxylic acid [intermediate 42] (98.2g, 569mmol) whilst maintaining the temperature of the reaction below 20°C. The mixture was stirred overnight at ambient temperature and then cooled to 0°C. Water (70ml_) was slowly added dropwise. After a further 5 minutes, aqueous potassium hydroxide solution (15% w/v, 70ml_) was slowly added, followed by additional water (200ml_). The mixture was allowed to warm to ambient temperature before magnesium sulfate was added and then stirred for 30 minutes. The mixture was then filtered, the filtered solid was washed with ethyl acetate (2000ml_) and the filtrate was evaporated to dryness to afford the crude product. The crude product was suspended in dichloromethane (500ml_), filtered and the filtered solid was dried to afford the title compound (80g, 0.506mol, 88% yield). LCMS (Method A): Rt 0.66 minutes; m/z 159 (MH+).
References:
WO2011/110575,2011,A1 Location in patent:Page/Page column 81
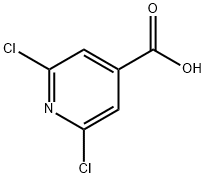
5398-44-7
322 suppliers
$6.00/1g

1334294-36-8
11 suppliers
inquiry