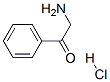
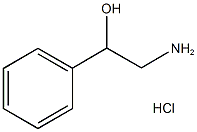
2-Aminoacetophenone hydrochloride synthesis
- Product Name:2-Aminoacetophenone hydrochloride
- CAS Number:5468-37-1
- Molecular formula:C8H10ClNO
- Molecular Weight:171.62
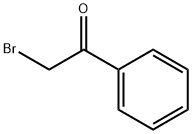
70-11-1
297 suppliers
$10.00/25g

5468-37-1
260 suppliers
$17.00/5G
Yield:5468-37-1 97%
Reaction Conditions:
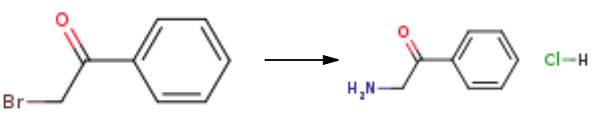
Stage #1:α-bromoacetophenone with hexamethylenetetramine in diethyl ether at 20; for 12 h;Delepine Amine Synthesis;
Stage #2: with hydrogenchloride;water in ethanol for 3 h;Reflux;
Steps:
General Procedure for Delpine Reaction.
General procedure: To a stirred solution of 2-methoxyphenacyl bromide (7a)/phenacyl bromide (7b) (1 mmol, 1 equiv) in diethyl ether (13 mL) was added hexamethylenetetramine (1 mmol, 1 equiv) all at once and the mixture was stirred for 12 h at room temperature (solid formation observed). The resulting solid was filtered, washed with diethyl ether (15 mL), and dried under reduced pressure to afford the quaternary salt, which was next placed in a two-necked round-bottomed flask fitted with reflux condenser and EtOH (22 mL) was added to it. Concentrated HCl (0.6 mL) was added to it and the mixture was refluxed for 3 h (solid formed). After cooling to room temperature, the solid was filtered, washed with EtOH (20 mL), and dried under vacuum to afford pure 2- methoxybenzoylmethylammonium chloride (8a)/benzoylmethylammonium chloride (8b) salt.
References:
Jang, Ha Young;Damodar, Kongara;Kim, Jin-Kyung;Jun, Jong-Gab [Bulletin of the Korean Chemical Society,2017,vol. 38,# 12,p. 1481 - 1485]
1816-88-2
5 suppliers
inquiry

5468-37-1
260 suppliers
$17.00/5G
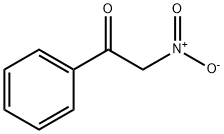
614-21-1
67 suppliers
$63.00/2 g

5468-37-1
260 suppliers
$17.00/5G

614-21-1
67 suppliers
$63.00/2 g

5468-37-1
260 suppliers
$17.00/5G
4561-43-7
8 suppliers
$116.64/250mgs:
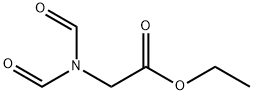
128038-38-0
0 suppliers
inquiry

5468-37-1
260 suppliers
$17.00/5G