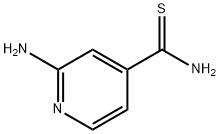
2-aminopyridine-4-carbothioamide synthesis
- Product Name:2-aminopyridine-4-carbothioamide
- CAS Number:88526-59-4
- Molecular formula:C6H7N3S
- Molecular Weight:153.2
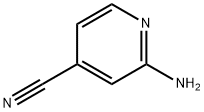
42182-27-4
250 suppliers
$10.00/1g

88526-59-4
8 suppliers
inquiry
Yield:88526-59-4 81%
Reaction Conditions:
with O,O-Diethyl hydrogen phosphorodithioate in tetrahydrofuran;water at 60; for 28 h;
Steps:
1-B.i
[00616] (i) Production of 2-aminopyridine-4-carbothioamide[00617] A mixture of 2-aminopyridine-4-carbonitrile (6.0 g, 50 mmol), O,O' -diethyl dithiophosphate (11 mL, 60 mmol), tetrahydrofuran (25 mL) and water (25 mL) was stirred at 600C for 4 hr. To the reaction mixture was added O,O'-diethyl dithiophosphate (2.8 mL, 15 mmol) and the mixture was stirred at 600C for 1 day. To the reaction mixture was added aqueous sodium bicarbonate solution, and the mixture was extracted with ethyl acetate. The combined organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate, and the insoluble material was filtered off. The filtrate was concentrated under reduced pressure, and the obtained residue was washed with diisopropyl ether to give the title compound (6.2 g, 81%) as a yellow solid.[00618] 1H-NMR (DMSO-dβ, 300 MHz) δ 6.12 (2H, s), 6.73 (IH, dd, J = 1.7, 5.3 Hz), 6.77 - 6.81 (IH, m), 7.92 (IH, dd, J = 0.6, 5.3 Hz), 9.53 (IH, br s), 9.95 (IH, br s).
References:
WO2010/90716,2010,A1 Location in patent:Page/Page column 251
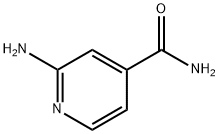
13538-42-6
87 suppliers
$37.00/1g

88526-59-4
8 suppliers
inquiry