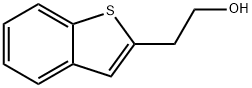
2-BENZO[B]THIOPHEN-2-YL-ETHANOL synthesis
- Product Name:2-BENZO[B]THIOPHEN-2-YL-ETHANOL
- CAS Number:30962-69-7
- Molecular formula:C10H10OS
- Molecular Weight:178.25
Yield:30962-69-7 79%
Reaction Conditions:
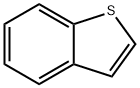
Stage #1: Benzo[b]thiophene in tetrahydrofuran;hexanes at -50 - -40; for 0.75 h;
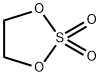
Stage #2: ethyleneglycol sulfate in tetrahydrofuran;hexanes at -40 - 20;
Stage #3: with acetyl chloride in methanol at 20; for 4.25 h;
Steps:
1B.2
EXAMPLE 1B; SYNTHESIS OF REAGENT (2-BENZO[B]THIOPHEN-2-YL-ETHYL)-DIMETHYL-AMINE, ROUTE 2; An alternative synthesis for compound 1a follows: Benzo[b]thiophene (20 g, 149 mmol) was dissolved in anhydrous THF (600 mL) and cooled to -50° C. N-BuLi (85 mL, 135 mmol, 1.6M in hexanes) was added and the mixture was stirred for 45 min at -40° C. A solution of [1,3,2]Dioxathiolane 2,2-dioxide (16.8 g, 135 mmol) in THF (100 mL) was added dropwise and the temperature was allowed to slowly reach room temperature, while being stirred overnight. Water and ether were added and the product was extracted with water. The combined water layers were concentrated in vacuo, MeOH and dichloromethane were added and the mixture was again concentrated in vacuo. In a separate vessel AcCl (70 mL) was slowly added to cooled (0° C.) MeOH (700 mL) and stirred for 15 min. This mixture was cannulated to the residue obtained as described above and stirred for 4 hrs at room temperature. After concentration, EtOAc was added and the mixture was washed with sat. NaHCO3 and brine, dried (Na2SO4), filtered and concentrated in vacuo to generate 19.1 g of 2-Benzo[b]thiophen-2-yl-ethanol as a white solid in 79% yield.
References:
US2006/14797,2006,A1 Location in patent:Page/Page column 16