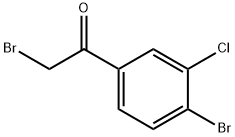
2-bromo-1-(4-bromo-3-chlorophenyl)ethanone synthesis
- Product Name:2-bromo-1-(4-bromo-3-chlorophenyl)ethanone
- CAS Number:87427-57-4
- Molecular formula:C8H5Br2ClO
- Molecular Weight:312.39
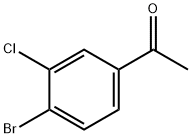
3114-31-6

87427-57-4
Copper dibromide (CuBr2, 955 mg, 4.18 mmol) and ethyl acetate (EtOAc, 10 mL) were added to a two-necked round-bottomed flask fitted with a reflux condenser tube under argon protection. The suspension was stirred for 20 min. Subsequently, 4'-bromo-3'-chloroacetophenone (500 mg, 2.14 mmol), which was pre-dissolved in ethyl acetate (EtOAc, 10 mL), was added to the reaction mixture and the reaction was heated at 80 °C for 1 hour. During the reaction, copper dibromide (CuBr2, 460 mg, 2.06 mmol) was added additionally and the reaction mixture was continued to be stirred at 80 °C overnight. Upon completion of the reaction, the suspension was cooled to room temperature, filtered through a diatomaceous earth pad and washed with ethyl acetate (EtOAc). The combined filtrates were washed with saturated sodium bicarbonate (NaHCO3) solution, the organic layer was dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. Purification by silica gel fast column chromatography (petroleum ether/ethyl acetate=4:1) afforded the target product 2-bromo-1-(4-bromo-3-chlorophenyl)ethanone (582 mg, 87%) as a yellow viscous oil. Nuclear magnetic resonance hydrogen spectrum (1H NMR, 300 MHz, CDCl3) δ 8.06 (d, J = 2.1 Hz, 1H, Har), 7.77 (d, J = 8.5 Hz, 1H, Har), 7.71 (dd, J = 8.4, 2.0 Hz, 1H, Har), 4.38 (s, 2H, CH2). Nuclear magnetic resonance carbon spectrum (13C NMR, 75 MHz, CDCl3) δ 134.7 (CH), 131.0 (CH), 128.3 (CH), 30.4 (CH2). High Resolution Mass Spectrometry (HRMS, ESI) [M + H]+ C8H6Br2ClO: Calculated value 310.8468, measured value 310.8455.

3114-31-6
79 suppliers
$9.00/250mg

87427-57-4
14 suppliers
inquiry
Yield:87427-57-4 87%
Reaction Conditions:
with copper(ll) bromide in ethyl acetate at 80;Inert atmosphere;
Steps:
2-bromo-1-(4-bromo-3-chlorophenyl)ethanone
In a two necked round bottom flask equipped with a reflux condenser under an argon atmosphere was added CuBr2 (955 mg, 4.18 mmol) and EtOAc (10 mL). The suspension was stirred during 20 minutes. The 4-bromo-3- methylacetophenonone (500 mg, 2.14 mmol) pre-dissolved in EtO Ac (10 mL) was added to the mixture and heated at 80°C during one hour. A new equivalent of CuBr2 (460 mg, 2.06 mmol) was added and the reaction was stirred at 80°C overnight. The suspension was cooled down, filtered through a Celite pad and washed with EtO Ac. The filtrate was washed with a saturated NaHCO3 solution. The organic layer was dried over Na2SO4, filtered and evaporated under vacuum. Purification by silica gel flash chromatography (PE/EtOAc 4: 1) afforded the product (582 mg, 87%) as a yellow viscous oil. NMR (300 MHz, CDC13) δ 8.06 (d, J = 2.1 Hz, 1H, Har), 7.77 (d, J = 8.5 Hz, 1H, Har), 7.71 (dd, J = 8.4, 2.0 Hz, 1H, Har), 4.38 (s, 2H, CH2). 13C NMR (75 MHz, CDC13) δ 134.7 (CH), 131.0 (CH), 128.3 (CH), 30.4 (CH2). HRMS (ESI) [M+H]+ C8H6Br2ClO: Calcd. 310.8468 found 310.8455.
References:
WO2015/1024,2015,A1 Location in patent:Page/Page column 73
21900-32-3
17 suppliers
$91.90/250mgs:

18107-18-1
210 suppliers
$33.00/5g

87427-57-4
14 suppliers
inquiry
1261859-54-4
6 suppliers
inquiry
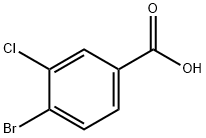
25118-59-6
208 suppliers
$6.00/1g

87427-57-4
14 suppliers
inquiry

1235711-34-8
6 suppliers
inquiry

87427-57-4
14 suppliers
inquiry