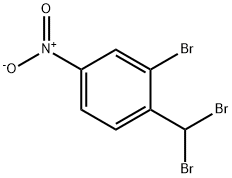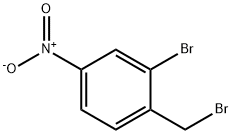
2-bromo-1-(bromomethyl)-4-nitrobenzene synthesis
- Product Name:2-bromo-1-(bromomethyl)-4-nitrobenzene
- CAS Number:940-05-6
- Molecular formula:C7H5Br2NO2
- Molecular Weight:294.93
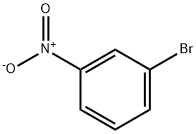
585-79-5

940-05-6
General procedure for the synthesis of 2-bromo-4-nitrobenzyl bromide from m-bromonitrobenzene: A mixture of 2-bromo-6-nitrobenzene (1.08 g, 5.00 mmol) and N-bromosuccinimide (908 mg, 5.10 mmol) was dissolved in carbon tetrachloride (15 mL), and α,α'-azobisisobutyronitrile (AIBN) (28 mg) was added as initiator. The reaction mixture was stirred at 70 °C for 3 hours. Subsequently, additional AIBN (82 mg) was added in batches at the same temperature and stirring was continued for 4 hours. After completion of the reaction, the reaction mixture was diluted with chloroform and filtered to remove the precipitate. The filtrate was distilled under reduced pressure to remove the solvent and the crude product obtained was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=1/1) to afford the target compound 2-bromo-1-(bromomethyl)-4-nitrobenzene (493 mg, 33% yield). The product was characterized by 1H-NMR (CDCl3): δ 4.62 (2H, s), 7.65 (1H, d, J=8.4Hz), 8.17 (1H, dd, J=8.4,2.2Hz), 8.46 (1H, d, J=2.2Hz).

585-79-5
30 suppliers
$15.00/1g

940-05-6
75 suppliers
inquiry
Yield: 33%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane at 70; for 3 h;
Steps:
75 2-Bromo-1-(bromomethyl)-4-nitrobenzene
To a mixture of 2-bromo-6-nitrobenzene (1.08g, 5.00 mmol) and N-bromosuccinimide (908mg, 5.10 mmol), α,α'-azobisisobutyronitrile (AIBN) (28mg) was added in carbon tetrachloride (15mL) at 70°C and stirred at 70°C for 3 hours. Further, AIBN (82mg) was added portionwise with stirring 70°C for 4 hours. After a dilution with chloroform, precipitate was filtered off and the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane/ethyl acetate = 1/1) to give 2-bromo-1-(bromomethyl)-4-nitrobenzene (493mg, 33%). 1H-NMR (CDCl3) δ: 4.62(2H,s), 7.65(1H,d,J=8.4Hz), 8.17(1H,dd,J=8.4, 2.2Hz), 8.46(1H,d,J=2.2Hz)
References:
Dainippon Sumitomo Pharma Co., Ltd. EP1844768, 2007, A1 Location in patent:Page/Page column 39
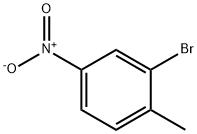
7745-93-9
293 suppliers
$5.00/5g

940-05-6
75 suppliers
inquiry