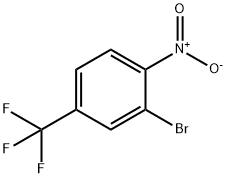
2-Bromo-1-nitro-4-(trifluoromethyl)benzene synthesis
- Product Name:2-Bromo-1-nitro-4-(trifluoromethyl)benzene
- CAS Number:132839-58-8
- Molecular formula:C7H3BrF3NO2
- Molecular Weight:270
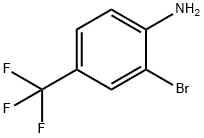
57946-63-1

132839-58-8
The general procedure for the synthesis of 2-bromo-4-trifluoromethylnitrobenzene (95% content) from 2-bromo-4-(trifluoromethyl)aniline was as follows: the compound was prepared by oxidizing 4-(trifluoromethyl)aniline. To a 300 mL three-necked round-bottomed flask equipped with a reflux condenser and a dropping funnel was added sodium perborate tetrahydrate (40.69 g, 0.264 mol) and glacial acetic acid (50 mL). 2-Bromo-4-(trifluoromethyl)aniline (6.31 g, 26.8 mmol) and glacial acetic acid (50 mL) were added to the dropping funnel. The suspension in the flask was heated to 55°C and the contents of the dropping funnel were slowly added dropwise.After 3 h, additional sodium perborate tetrahydrate (20.00 g, 0.130 mol) was added to the reaction mixture and stirring was continued at 55°C for 4 h. The reaction was carried out at 55°C. The yellow suspension obtained from the reaction was cooled to room temperature and filtered through a diatomaceous earth pad. The organic phase was separated by adding ether (200 mL) and water (100 mL) to the filtrate. The aqueous phase was extracted twice with diethyl ether (150 mL x 2). The combined organic phases were separated by neutralization with 3M aqueous sodium hydroxide solution. The separated organic phase was dried with sodium sulfate and then concentrated to give a brown liquid. The crude product was purified by fast column chromatography (12% EtOAc/hexane) to give 2-bromo-4-trifluoromethylnitrobenzene (95% content) as a final yellow liquid (4.22 g, 60% yield).

57946-63-1
238 suppliers
$10.00/5g

132839-58-8
75 suppliers
$13.00/250mg
Yield: 60%
Reaction Conditions:
with sodium perborate tetrahydrate;acetic acid at 55; for 7 h;
Steps:
Preparation of 2-bromo-1-nitro-4-(trifluoromethyl)benzene
This compound was prepared by application of the procedure for oxidation of4-(trifluoromethyl)aniline.[7] A 300 mL three-necked round-bottom flask equipped with a refluxcondenser and a dropping funnel was charged with sodium perborate tetrahydrate (40.69 g, 0.264mol) and glacial acetic acid (50 mL). The dropping funnel was charged with2-bromo-4-(trifluoromethyl)aniline (6.31 g, 26.8 mmol) and glacial acetic acid (50 mL). Thesuspension in the flask was heated to 55°C and the content of the funnel was added dropwise to it.After 3 h, an additional sodium perborate tetrahydrate (20.00 g, 0.130 mol) was added to the reactionmixture and stirred at 55°C for 4 h. The resulting yellow suspension was cooled to room temperatureand filtered through a Celite pad. To the filtrate was added diethyl ether (200 mL) and water (100mL). The organic phase was separated from the mixture and aqueous phase was extracted withdiethyl ether (150 mL x 2). Combined organic phase was neutralized by aqueous sodium hydroxidesolution (3 M) and separated. The separated organic phase was dried over sodium sulfate andconcentrated to afford brown liquid. This crude product was purified by flash columnchromatography (12% EtOAc/hexane) to give the title compound as a yellow liquid (4.22 g, 60 %).
References:
Takahashi, Hirotsugu;Watanabe, Takahito;Tobita, Hiromi [Chemistry Letters,2018,vol. 47,# 3,p. 296 - 299] Location in patent:supporting information
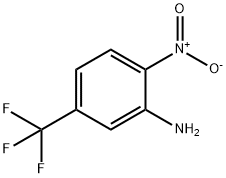
402-14-2
105 suppliers
$20.00/250mg

132839-58-8
75 suppliers
$13.00/250mg