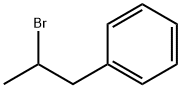
2-BROMO-1-PHENYLPROPANE synthesis
- Product Name:2-BROMO-1-PHENYLPROPANE
- CAS Number:2114-39-8
- Molecular formula:C9H11Br
- Molecular Weight:199.09
![Benzoic acid, 2-[(1-methyl-2-phenylethoxy)sulfonyl]-, 2-(2-methoxyethoxy)ethyl ester](/CAS/20210305/GIF/866942-23-6.gif)
866942-23-6
0 suppliers
inquiry

2114-39-8
131 suppliers
$45.00/50mg
Yield:2114-39-8 96%
Reaction Conditions:
with lithium bromide in acetone; for 2 h;Product distribution / selectivity;Heating / reflux;
Steps:
Table 2. Comparison of the rates of lithium, sodium, and potassium bromide additions to various alkyl sulfonates. EPO
References:
WO2006/60142,2006,A2 Location in patent:Page/Page column 15-16
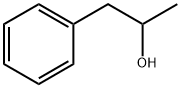
698-87-3
119 suppliers
$10.00/1g

2114-39-8
131 suppliers
$45.00/50mg

14135-71-8
1 suppliers
inquiry

2114-39-8
131 suppliers
$45.00/50mg
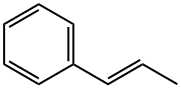
873-66-5
121 suppliers
$35.00/250mg

2114-39-8
131 suppliers
$45.00/50mg
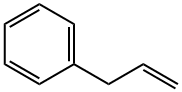
300-57-2
231 suppliers
$53.00/25mL

2114-39-8
131 suppliers
$45.00/50mg