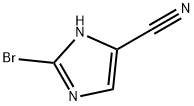
2-bromo-1H-Imidazole-5-carbonitrile synthesis
- Product Name:2-bromo-1H-Imidazole-5-carbonitrile
- CAS Number:1067894-55-6
- Molecular formula:C4H2BrN3
- Molecular Weight:171.98
1067894-54-5
1 suppliers
inquiry

1067894-55-6
6 suppliers
inquiry
Yield:1067894-55-6 61.2%
Reaction Conditions:
with cyclohexyl acetate;sodium iodide at 20 - 105;Inert atmosphere;
Steps:
10
[Example 10]; While a mixture of 25.1 g (100.0 mmol) of 4-cyano-2,5-dibromoimidazole and 42.7 g (300.0 mmol) of cyclohexyl acetate was stirred at ordinary temperature, 22.5 g (150.0 mmol) of sodium iodide was added thereto to prepare a reaction liquid. After the reaction liquid was stirred at 105°C for 12 hours under a nitrogen stream, the liquid was stirred under ice cooling for 1 hour. When 2-bromo-4-cyanoimidazole in the reaction liquid was analyzed by means of HPLC, it was confirmed that the product was formed in an amount of 11.2 g (65.3 mmol, conversion: 65.3%). After the reaction liquid was concentrated under reduced pressure, the product was purified by silica gel chromatography (ethyl acetate/hexane system) and, after volatile matter was further vaporized to solidify the product, crystals of 2-bromo-4-cyanoimidazole were obtained in an amount of 10.5 g (yield: 61.2%) as a residue. Incidentally, the NMR spectrum of the crystals was measured and was compared with the NMR spectrum of its specimen, whereby it was confirmed that the product is 2-bromo-4-cyanoimidazole.
References:
EP2141151,2010,A1 Location in patent:Page/Page column 8