
2-BROMO-3,4,5-TRIMETHOXY-BENZALDEHYDE synthesis
- Product Name:2-BROMO-3,4,5-TRIMETHOXY-BENZALDEHYDE
- CAS Number:35274-53-4
- Molecular formula:C10H11BrO4
- Molecular Weight:275.1
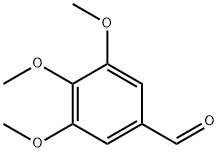
86-81-7
487 suppliers
$16.26/10gm:

35274-53-4
27 suppliers
$61.60/1g
Yield:35274-53-4 98%
Reaction Conditions:
with bromine in chloroform;water;Heating / reflux;
Steps:
1
The (4,4',5,6-tetramethoxybiphenyl-2,2'-diyl)dimethanol starting material was made as follows:To a stirred refluxing solution of 3,4,5-trimethoxybenzaldehyde (17.0 g, 86.6 mmol) in water (3 mL) and chloroform (100 mL) was added a solution of bromine (14.7 g, 92 mmol) in chloroform (30 mL) dropwise. The solution was heated under reflux overnight, cooled, washed with water (2 x 100 mL) and saturated aqueous sodium hydrogen carbonate (50 mL), dried over MgSO4, filtered and evaporated. The residual orange oil (27 g) crystallised on standing. The solid was washed with petroleum ether, b.p. 40-60 0C, to obtain 2-bromo-3,4,5-trimethoxybenzaldehyde as white crystals (23.4 g, 98%); m.p. 69 °C (literature value 69.5-71°C, see Brown, E.; Robin, J.-P.; Dhal, R. Tetrahedron 1982, 38, 2569-2579); NMR Spectrum δH (300 MHz, CDCl3) 10.40 (1 H, s, 1-CHO), 7.29 (1 H, s, 6-H), 4.00 (3 H, s, OCH3), 3.95 (3 H, s, OCH3) and 3.93 (3 H, s, OCH3); NMR Spectrum δc (75 MHz, CDCl3) 56.6, 61.6, 106.9, 126.1, 128.1, 149.2, 150.3, 152.8, 189.2; v^/cin"1 (thin film) 2940, 2843, 1685 (C=O), 1588, 1480, 1460, 1386, 1317, 1196, 1161, 1134, 1103 and 1002 (data in accordance with published values, see Brown, E.; Robin, J.-P.; Dhal, R. Tetrahedron 1982, 38, 2569-2579); Rf (hexane - ethyl acetate 4:1) 0.50.
References:
WO2008/75048,2008,A2 Location in patent:Page/Page column 64

73252-54-7
1 suppliers
inquiry

35274-53-4
27 suppliers
$61.60/1g
3840-31-1
215 suppliers
$33.00/25g

35274-53-4
27 suppliers
$61.60/1g

70242-11-4
1 suppliers
inquiry

35274-53-4
27 suppliers
$61.60/1g
23346-82-9
24 suppliers
$28.60/5MG

35274-53-4
27 suppliers
$61.60/1g