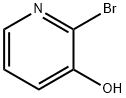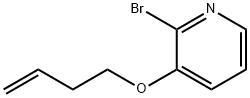
2-Bromo-3-(but-3-enyloxy)pyridine synthesis
- Product Name:2-Bromo-3-(but-3-enyloxy)pyridine
- CAS Number:405174-45-0
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
Yield:405174-45-0 88%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran at 50; for 6 h;Inert atmosphere;
Steps:
2.1 Synthesis of S2
The compound 2-bromo-3-hydroxypyridine (20 g, 1.0 eq, 115 mmol), 4-hydroxy-1-butene (8.29 g, 1.0 eq, 115 mmol) and triphenylphosphine (36.2 g, 1.2) were added to a three-neck flask. Eq, 138 mmol), after dissolving in 150 ml of anhydrous tetrahydrofuran under nitrogen atmosphere, stirring for a while, and then dropping DEAD (22 g, 1.1 eq, with a constant pressure dropping funnel).126.5 mmol). After the dropwise addition, the oil bath was stirred at 50 ° C. After six hours, the plate was plated and the TLC test was complete. After the reaction was quenched with water, ethyl acetate was evaporated, washed with brine and dried over anhydrous Na? Filtration, the filtrate was dried under reduced pressure, and then purified by column chromatography ( petroleum ether: ethyl acetate = 25:1 → petroleum ether:Ethyl acetate = 4:1) gave a yellow solid, S2, yield 88%.
References:
CN109651357,2019,A Location in patent:Paragraph 0091; 0092-0095