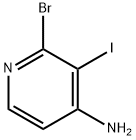
2-broMo-3-iodopyridin-4-aMine synthesis
- Product Name:2-broMo-3-iodopyridin-4-aMine
- CAS Number:1300750-77-9
- Molecular formula:C5H4BrIN2
- Molecular Weight:298.91
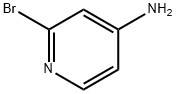
7598-35-8
362 suppliers
$6.00/250mg

1300750-77-9
34 suppliers
$45.00/10mg
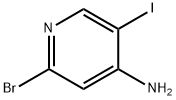
1300750-78-0
41 suppliers
inquiry
Yield:1300750-78-0 38% ,1300750-77-9 37%
Reaction Conditions:
with sodium acetate;Iodine monochloride in acetic acid at 75; for 3 h;
Steps:
5
2-Bromo-5-iodopyridin-4-amine (5); [00192] 4-Amino-2-bromopyridine (22.8g, 131 .8mmole) and sodium acetate (20.8g 254mmole) were stirred in acetic acid (82ml_) and a solution of iodine monochloride (1 M in acetic acid, 134ml_, 134 mmole) was added. The mixture was stirred and heated at 75°C for 3 hours. Most of the acetic acid was evaporated and the residue was partitioned between water (500ml_) and ethyl acetate (550ml_). The aqueous was again extracted with ethyl acetate (300ml_) The combined extracts were washed twice with 10% sodium carbonate solution (600, 300ml_), with 10% sodium thiosulfate solution (200ml_), with water and with brine, then dried and evaporated. This gave 40.3g of a crude product mix. This was combined with the crude product from a reaction on 7.5g of 4-amino-2- bromopyridine for purification. A large silica column (9cm internal diameter with 28cm bed of silica) was prepared in 5% ethyl acetate in dichloromethane. The crude material was applied in the same solvent. The column was eluted with 5% ethyl acetate in dichloromethane, with 10% ethyl acetate in dichloromethane and with 20% ethyl acetate in dichloromethane to give the desired isomer 5 (20.2g, 38%): 1 H-NMR (CDCI3, 500MHz): δ 4.74 (br s, 2H, NH2), 6.80 (s, 1 H), 8.34 (s, 1 H); and subsequently with 1 :1 ethyl acetate:dichloromethane to give 6 undesired isomer: 2-bromo-3-iodopyridin-4- amine 6 (19.3g, 37%).
References:
WO2012/123745,2012,A1 Location in patent:Page/Page column 65-66

7598-35-8
362 suppliers
$6.00/250mg

1300750-77-9
34 suppliers
$45.00/10mg

1300750-78-0
41 suppliers
inquiry

1377578-93-2
15 suppliers
inquiry