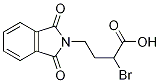
2-BroMo-4-(1,3-dioxoisoindolin-2-yl)butanoic acid synthesis
- Product Name:2-BroMo-4-(1,3-dioxoisoindolin-2-yl)butanoic acid
- CAS Number:35197-64-9
- Molecular formula:C12H10BrNO4
- Molecular Weight:312.12
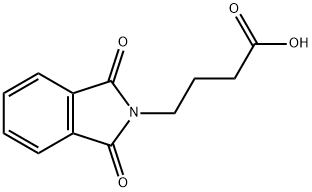
3130-75-4

35197-64-9
GENERAL STEPS: The synthesis of 2-bromo-4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)butanoic acid from 4-(1,3-dioxoisoindolin-2-yl)butanoic acid was carried out as follows: 4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)butanoic acid (3.00 g, 12.9 mmol) was mixed with thionyl chloride (20 mL ) were mixed and heated to reflux for 2 hours. Subsequently, bromine (2.06 g, 12.9 mmol) was added slowly over 4 hours while maintaining reflux. The reaction mixture was continued to be heated to reflux until complete consumption of the feedstock was confirmed by LC-MS, which took about 48 hours. Upon completion of the reaction, the excess thionyl chloride was removed under reduced pressure. Crushed ice was added to the residue and allowed to stand overnight. The precipitated white solid was filtered and dried in a vacuum oven to give 4.01 g of crude product, which could be used directly in subsequent steps without further purification. The product was confirmed by 1H NMR (270 MHz, methanol-D4): δ 2.19-2.38 (m, 1H), 2.39-2.58 (m, 1H), 3.83 (t, J=6.68 Hz, 2H), 4.37 (t, J=7.18 Hz, 1H), 7.73-7.91 (m, 4H). hplc analysis showed a purity of 71%. Retention time (Rt) was 1.12 min (System A, 30-80% acetonitrile, 3 min). Mass spectrometry (ESI+) analysis gave m/z 312 (M+H)+.

3130-75-4
167 suppliers
$24.00/100mg

35197-64-9
38 suppliers
$80.00/250mg
Yield:35197-64-9 96%
Reaction Conditions:
with phosphorus;bromine in tetrachloromethane;Heating;6-8 h;
References:
Kolasa, Teodozyj;Miller, Marvin J. [Journal of Organic Chemistry,1990,vol. 55,# 14,p. 4246 - 4255]
22509-74-6
241 suppliers
$9.00/5g

35197-64-9
38 suppliers
$80.00/250mg
85-44-9
780 suppliers
$9.00/5g

35197-64-9
38 suppliers
$80.00/250mg

108749-42-4
0 suppliers
inquiry

35197-64-9
38 suppliers
$80.00/250mg