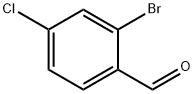
2-Bromo-4-chlorobenzaldehyde synthesis
- Product Name:2-Bromo-4-chlorobenzaldehyde
- CAS Number:84459-33-6
- Molecular formula:C7H4BrClO
- Molecular Weight:219.46
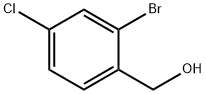
143888-84-0

84459-33-6
General procedure for the synthesis of 4-chloro-2-bromobenzaldehyde from (2-bromo-4-chlorophenyl)methanol: Pyridinium chlorochromate (432 mg, 2 mmol) was added to a solution of 2-bromo-4-chlorobenzyl alcohol (200 mg, 0.9 mmol) in dichloromethane (4 mL). The reaction mixture was stirred at room temperature for 2 hours. After the reaction was complete, ether (4 mL) was added and stirring was continued for 20 minutes. The mixture was poured into ether and the remaining solid was washed twice with ether. All ether solutions were combined and concentrated under reduced pressure. The product was purified by silica gel column chromatography with an eluent ratio of 20:80 to 1:1 ethyl acetate:dichloromethane to give 4-chloro-2-bromobenzaldehyde as a white solid (167 mg, 85% yield).

143888-84-0
35 suppliers
$150.00/1g

84459-33-6
193 suppliers
$7.00/5g
Yield:84459-33-6 85%
Reaction Conditions:
with pyridinium chlorochromate in dichloromethane; for 2 h;
Steps:
83
Add pyridinium chlorochromate (432 mg, 2 mmol) to a solution of 2-bromo-4- chlorobenzyl alcohol (200 mg, 0.9 mmol) in dichloromethane (4 mL). Stir the resulting mixture for 2 h. Add diethyl ether (4 mL) and stir for 20 "min. Decant the diethyl ether solution. Wash the remaining solids with diethyl ether twice. Combine the diethyl ether solutions and concentrate. Chromatograph on silica gel, eluting with 20:80 to 1:1 ethyl acetate:dichloromethane to give 2-bromo-4-chlorobenzaldehyde as a white solid (167 mg, 85%).
References:
WO2006/44454,2006,A1 Location in patent:Page/Page column 47

1202889-66-4
1 suppliers
inquiry

84459-33-6
193 suppliers
$7.00/5g
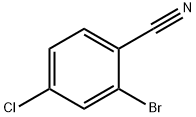
57381-49-4
88 suppliers
inquiry

84459-33-6
193 suppliers
$7.00/5g
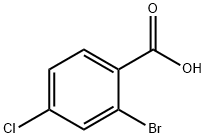
936-08-3
249 suppliers
$11.00/5g

84459-33-6
193 suppliers
$7.00/5g
27139-97-5
239 suppliers
$6.00/5g

84459-33-6
193 suppliers
$7.00/5g