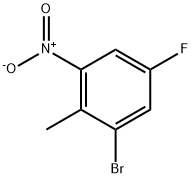
2-BROMO-4-FLUORO-6-NITROTOLUENE synthesis
- Product Name:2-BROMO-4-FLUORO-6-NITROTOLUENE
- CAS Number:502496-33-5
- Molecular formula:C7H5BrFNO2
- Molecular Weight:234.02
446-10-6

502496-33-5
Step 23a: 3-bromo-5-fluoro-2-methylaniline (Compound 0104-69) was added to a solution of trifluoroacetic acid (40 mL) containing 4-fluoro-2-nitrotoluene (10.0 g, 64.4 mmol). Subsequently, sulfuric acid (12.5 mL) was added slowly and N-bromosuccinimide (NBS, 17.2 g, 96.6 mmol) was added in batches. The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the mixture was slowly poured into ice water and stirring was continued for 15 min. Extraction was carried out with ethyl acetate, the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the target product 1-bromo-5-fluoro-2-methyl-3-nitrobenzene (15.0 g, 100% yield) as a yellow oil. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 2.41 (s, 3H), 7.96 (dd, J = 8.0,2.4Hz, 1H), 8.02 (dd, J = 8.0,2.4Hz, 1H).

446-10-6
334 suppliers
$10.00/25g

502496-33-5
164 suppliers
inquiry
Yield:502496-33-5 100%
Reaction Conditions:
with N-Bromosuccinimide;sulfuric acid;trifluoroacetic acid at 20; for 16 h;
Steps:
23.23a
Step 23a: 3-Bromo-5-fluoro-2-methylbenzenamine (Compound 0104-69)To a solution of 4-fluoro-2-nitrotoluene (10.0 g, 64.4 mmol) in trifluoroacetic acid (40 mL) was added con. sulfuric acid (12.5 mL) followed by NBS (17.2 g, 96.6 mmol) and the reaction mixture was stirred at room temperature for 16 h. Then the reaction mixture was poured into ice and water and stirred for 15 min. Extracted with ethyl acetate and the organic layer was washed with brine, dried, concentrated to get compound l-bromo-5- fluoro-2-methyl-3 -nitrobenzene (15.0 g, 100%) as a yellow oil. 1H NMR (400 MHz, OMSO-d6) δ 2.41 (s, 3H), 7.96 (dd, J= 8.0, 2.4 Hz, 1H), 8.02 (dd, J= 8.0, 2.4 Hz, 1H).
References:
WO2011/130628,2011,A1 Location in patent:Page/Page column 155