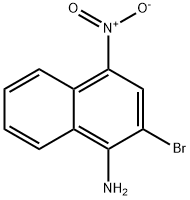
2-Bromo-4-nitro-naphthalen-1-ylamine synthesis
- Product Name:2-Bromo-4-nitro-naphthalen-1-ylamine
- CAS Number:63240-26-6
- Molecular formula:C10H7BrN2O2
- Molecular Weight:267.08
776-34-1
165 suppliers
$8.00/1g

63240-26-6
36 suppliers
inquiry
Yield:63240-26-6 96%
Reaction Conditions:
with N-Bromosuccinimide;ammonium acetate in acetonitrile at 20; for 0.166667 h;
Steps:
2-Bromo-4-nitronaphthalen-1-amine (34)
To a solution of 4-nitronaphthalen-1-amine (1.0 g, 5.31 mmol) in acetonitrile (17 mL) were added ammonium acetate (41 mg, 0.53 mmol) andN-bromosuccinimide(993 mg, 5.58 mmol) at room temperature. The reaction mixture was stirred at room temperature for 10 minutes. After the reaction was completed, the crude mixture was concentrated under reduced pressure, added to water,and then extracted with ethyl acetate. The organic layer was dried over anhydrousNa2SO4andconcentrated under reduced pressure. The resulting solid was dissolved with the minimum amount of dichloromethane, solidified from hexane, and then filtered to give the product as a yellow solid (1.4 g, 96%);1H NMR (400 MHz,DMSO-d6)δ8.78 (d,J= 8.8 Hz, 1H), 8.54 (s, 1H), 8.45 (d,J= 8.8 Hz, 1H), 7.79-7.75 (m, 1H), 7.62-7.58 (m, 1H), 7.51 (brs, 2H, NH);13C NMR (100 MHz, DMSO-d6)δ149.5, 132.8, 131.9, 130.3, 126.24, 126.21, 123.7, 123.5, 121.0, 97.6;LC/MS (ESI+)m/z267.9 [M(79Br) + H]+, 269.6 [M(81Br) + H]+.
References:
Abed, Dhulfiqar Ali;Hu, Longqin;Lee, Sumi;Nguyen, Mai-Uyen;Verzi, Michael P. [European Journal of Medicinal Chemistry,2022,vol. 237,art. no. 114380] Location in patent:supporting information
575-36-0
110 suppliers
$32.00/25g

63240-26-6
36 suppliers
inquiry