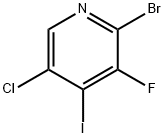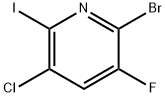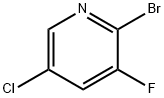
2-Bromo-5-chloro-3-fluoropyridine synthesis
- Product Name:2-Bromo-5-chloro-3-fluoropyridine
- CAS Number:514797-97-8
- Molecular formula:C5H2BrClFN
- Molecular Weight:210.43
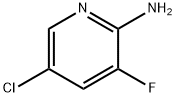
246847-98-3

514797-97-8
Step 1: 2-Amino-3-fluoro-5-chloropyridine (5.0 g, 34 mmol) was slowly added to 48% hydrobromic acid (HBr) solution (20 mL) at 0°C with stirring. Bromine (Br2, 5.24 mL, 102.3 mmol) was then added dropwise to the resulting mixture over 20 min at 0 °C. The reaction mixture was cooled to -10°C. A solution of sodium nitrite (NaNO2, 5.88 g, 85.3 mmol) in water (20 mL) was slowly added to the mixture over a period of 1.5 h and the mixture continued to be stirred for 30 min. Subsequently, a solution of sodium hydroxide (NaOH, 12 g, 306 mmol) in water (20 mL) was added over 30 minutes and the mixture was gradually warmed to room temperature. After completion of the reaction, the mixture was extracted with ether (3 x 100 mL). The combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to give the title compound 2-bromo-5-chloro-3-fluoropyridine as a pale yellow solid (6.43 g, 90% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 2.1 Hz, 1H), 7.48 (dd, J = 7.1,2.1 Hz, 1H).

246847-98-3
124 suppliers
$6.00/1g

514797-97-8
137 suppliers
$4.00/1g
Yield: 90%
Reaction Conditions:
with hydrogen bromide;bromine at 0; for 0.333333 h;
Steps:
1.16.1 Step 1: 2-bromo-5-chloro-3-fluoropyridine
Step 1: 2-bromo-5-chloro-3-fluoropyridine [0218] 5-chloro-3-fluoropyridin-2-amine (5.0 g, 34 mmol) was slowly added to 48% HBr solution (20 mL) with stirring at 0°C. To the resulting mixture E (5.24 mL, 102.3 mmol) was then added over 20 minutes at 0°C. The reaction mixture was cooled to -10°C. A solution of NaNC"2 (5.88 g, 85.3 mmol) in water (20 mL) was added over 1.5 hours, and the mixture stirred for additional 30 minutes. A solution of NaOH (12 g, 306 mmol) in water (20 mL) was added over 30 minutes, and the mixture was allowed to warm to room temperature. The mixture was extracted with ether (3x 100 mL). The combined organic phases were washed with brine, dried over sodium sulfate, filtered, and concentrated to afford the title compound as pale yellow solid (6.43 g, 90%). 1H NMR (400 MHz, CDC13) δ 8.23 (d, J = 2.1 Hz, 1H), 7.48 (dd, J= 7.1, 2.1 Hz, 1H).
References:
RUGEN HOLDINGS (CAYMAN) LIMITED;SHAPIRO, Gideon WO2015/187845, 2015, A1 Location in patent:Paragraph 0218
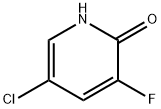
514797-96-7
82 suppliers
$18.00/250mg

514797-97-8
137 suppliers
$4.00/1g

514797-96-7
82 suppliers
$18.00/250mg

514797-97-8
137 suppliers
$4.00/1g
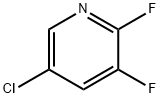
89402-43-7
389 suppliers
$6.00/5g

514797-97-8
137 suppliers
$4.00/1g