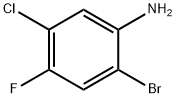
2-Bromo-5-chloro-4-fluoroaniline synthesis
- Product Name:2-Bromo-5-chloro-4-fluoroaniline
- CAS Number:85462-59-5
- Molecular formula:C6H4BrClFN
- Molecular Weight:224.46
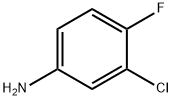
367-21-5

85462-59-5
General procedure for the synthesis of 2-bromo-4-fluoro-5-chloroaniline from 3-chloro-4-fluoroaniline: to a solution of 3-chloro-4-fluoroaniline (5 g, 34.3 mmol) in dichloromethane (60 mL) under ice-bath cooling conditions was added pyridine (4.2 ml, 51.5 mmol), and bromine (1.8 ml, 34.3 mmol) was added slowly and dropwise to a dichloromethane ( 40mL) solution. The reaction mixture was stirred continuously for 1 hour under an ice bath. Upon completion of the reaction, the reaction was quenched by adding aqueous sodium thiosulfate to the mixture and extracted with ethyl acetate. The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane) to afford the target product 2-bromo-4-fluoro-5-chloroaniline (6.4 g, yield: 83%) as an orange solid. m/z = 223 [M + H]+ by LC-MS.

367-21-5
523 suppliers
$5.00/25g

85462-59-5
79 suppliers
$12.00/250mg
Yield:85462-59-5 83%
Reaction Conditions:
with pyridine;bromine in dichloromethane; for 1 h;Cooling with ice;
Steps:
25.1 Example 25: Preparation of Compound 59Step 1
To a solution of Compound 56 (5g, 34.3mmol) in dichloromethane (60mL) was added pyridine (4.2ml, 51.5mmol)under ice-cooling, dropwised a solution of bromine (1.8ml, 34.3mmol) in dichloromethane (40mL), the solution wasstirred for 1 hour. To the reaction mixture was added sodium thiosulfate aqueous solution, and extracted with ethylacetate. The organic layer was dried with anhydrous sodium sulfate, and concentrated under reduced pressure. Theresidue was purified by silica gel chromatography (ethyl acetate / hexane) to yield Compound 57 (6.4g, yield: 83%) asan orange solid.LC-MS: m/z=223. [M+H]+
References:
EP3112369,2017,A1 Location in patent:Paragraph 0296; 0297