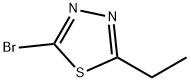
2-BROMO-5-ETHYL-1,3,4-THIADIAZOLE synthesis
- Product Name:2-BROMO-5-ETHYL-1,3,4-THIADIAZOLE
- CAS Number:57709-49-6
- Molecular formula:C4H5BrN2S
- Molecular Weight:193.06
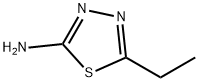
14068-53-2
138 suppliers
$13.43/250mgs:

57709-49-6
25 suppliers
$45.00/10mg
Yield:57709-49-6 50%
Reaction Conditions:
with n-Amyl nitrite;copper(ll) bromide in ISOPROPYLAMIDE;acetonitrile at 20; for 2 h;
Steps:
20.1
To a solution of 2-amino-5-ethyl-1,3,4-thiadiazole (1.00 g) in acetonitrile (20 mL)/dimethylacetamide (20 mL) were added copper (II) bromide (2.07 g) and n-amyl nitrite (1.40 mL), and the mixture was stirred at room temperature for 2 hours. The reaction mixture was concentrated under reduced pressure, and to the residue was added a saturated aqueous ammonium chloride solution, and then the mixture was extracted with ethyl acetate. The organic layer was washed successively with water and brine, dried over magnesium sulfate and then filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was purified by column chromatography on silica gel (solvent; hexane/ethyl acetate = 95/5 to 80/20) to give 2-bromo-5-ethyl-1,3,4-thiadiazole (0.75 g) as a colorless liquid (yield: 50%). MS(APCI)m/z; 193/195[M+H]+.
References:
EP2390254,2011,A1 Location in patent:Page/Page column 61-62