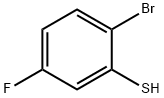
2-bromo-5-fluorobenzenethiol synthesis
- Product Name:2-bromo-5-fluorobenzenethiol
- CAS Number:55389-14-5
- Molecular formula:C6H4BrFS
- Molecular Weight:207.06
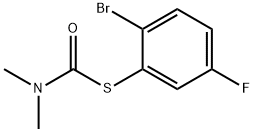
147460-42-2
2 suppliers
inquiry

55389-14-5
14 suppliers
$94.25/1g
Yield: 99%
Reaction Conditions:
with potassium hydroxide in methanol for 3 h;Heating / reflux;
Steps:
16 Experimentals for Compound 13 of Scheme 19
Example 16 Experimentals for Compound 13 of Scheme 19 [0206] A mixture of compound 20 (4.3 g, 15.5 mmol) and powdered KOH (4.3 g, 89.7 mmol) in methanol (300 mL) was heated to reflux for 3 hours. The solvent was removed under vacuum, and the residue was portioned between water (50 mL) and methylene chloride (50 mL). The aqueous layer was separated, acidified to pH 3 with 6 N HCl and reextracted with methylene chloride (3×100 mL). The organic layers were dried over Na2SO4 and concentrated to dryness to give the desired product 13 as pale yellow oil (3.3 g, 99%): 1H NMR (300 MHz, CDCl3) δ 7.50-7.45 (m 1H), 7.18-7.10 (m, 1H), 6.75-6.71 (m, 1H), 4.08 (s, 1H) ppm.
References:
Haydar, Simon N. US2003/225069, 2003, A1 Location in patent:Page 14, 24
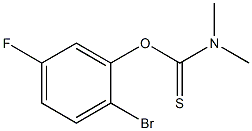
147460-40-0
2 suppliers
inquiry

55389-14-5
14 suppliers
$94.25/1g
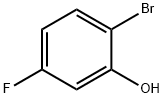
147460-41-1
261 suppliers
$5.00/1g

55389-14-5
14 suppliers
$94.25/1g