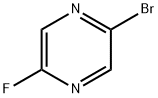
2-Bromo-5-fluoropyrazine synthesis
- Product Name:2-Bromo-5-fluoropyrazine
- CAS Number:1209459-10-8
- Molecular formula:C4H2BrFN2
- Molecular Weight:176.97
374063-92-0

1209459-10-8
The general procedure for the synthesis of 2-bromo-5-fluoropyrazine using 2-bromo-5-hydroxypyrazine as starting material was as follows: 4.2 g (24 mmol) of 2-bromo-5-hydroxypyrazine was dissolved in 25 mL of pyridine and cooled to 0 °C in an ice bath. Subsequently, 8.12 g (28.8 mmol) of trifluoromethanesulfonic anhydride (Aldrich) was added in batches over about 5 minutes. The reaction mixture was stirred in an ice bath for 30 minutes, followed by stirring at room temperature overnight. Upon completion of the reaction, the reaction mixture was mixed with 300 mL of ether and 500 mL of aqueous 1N HCl. The organic and aqueous layers were separated and the aqueous layer was back-extracted with 200 mL of ether. The combined organic phases were washed sequentially with saturated aqueous NaHCO3 solution (2 x 100 mL) and saturated aqueous NaCl solution (200 mL). The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate/hexane) to afford 5.55 g (75% yield) of 2-bromo-5-fluoropyrazine. The structure of the product was confirmed by 1H NMR.

374063-92-0
221 suppliers
$7.00/250mg

1209459-10-8
61 suppliers
$32.00/100mg
Yield: 75%
Reaction Conditions:
with trifluoromethylsulfonic anhydride in pyridine at 0 - 20;
Steps:
27.C Part C
4.2 g (24 mmol) of the product from Part B was dissolved in pyridine (25 mL) and cooled to 0° C. in an ice bath. Triflic anhydride (Aldrich, 8.12 g, 28.8 mmol) was added in several portions over approximately 5 min. The mixture was allowed to mix, stoppered with a syringe needle vent in an ice bath for approximately 30 min, and then stoppered overnight at ambient temperature. The reaction mixture with diethyl ether (300 mL) and aqueous 1N HCl (500 mL). Separated layers and back-extracted the aqueous layer with diethyl ether (200 mL). The combined organic extracts were washed with saturated aqueous NaHCO3 (2?100 mL ) and saturated aqueous NaCll (200 mL). The organic layers were dried over MgSO4, filtered, and concentrated in vacuo to an oil that was purified by chromatography (silica, ethyl acetate/hexanes) to produce 5.55 g (75%) of the desired product. 1H NMR confirmed the structure of the product.
References:
Barta, Thomas E.;Becker, Daniel P.;Bedell, Louis J.;Boehm, Terri L.;Brown, David L.;Carroll, Jeffery N.;Chen, Yiyuan;Fobian, Yvette M.;Freskos, John N.;Gasiecki, Alan F.;Grapperhaus, Margaret L.;Heintz, Robert M.;Hockerman, Susan L.;Kassab, Darren J.;Khanna, Ish K.;Kolodziej, Stephen A.;Massa, Mark A.;McDonald, Joseph J.;Mischke, Brent V.;Mischke, Deborah A.;Mullins, Patrick B.;Nagy, Mark A.;Norton, Monica B.;Rico, Joseph G.;Schmidt, Michelle A.;Stehle, Nathan W.;Talley, John J.;Vernier, William F.;Villamil, Clara I.;Wang, Lijuan J.;Wynn, Thomas A. US2005/9838, 2005, A1 Location in patent:Page 150