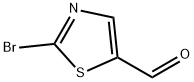
2-Bromo-5-fomylthiazole synthesis
- Product Name:2-Bromo-5-fomylthiazole
- CAS Number:464192-28-7
- Molecular formula:C4H2BrNOS
- Molecular Weight:192.03
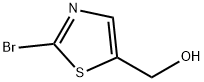
687636-93-7

464192-28-7
General procedure for the synthesis of 2-bromo-5-formylthiazole from 2-bromothiazole-5-methanol: Manganese dioxide (3.8 g, 37.10 mmol) was added to a solution of 2-bromothiazole-5-methanol (1.44 g, 7.42 mmol) in trichloromethane (20 mL). The reaction mixture was stirred at room temperature for 3 days. Upon completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure to give 2-bromo-5-formylthiazole (870 mg, 61% yield) as a white solid. The structure of the product was confirmed by nuclear magnetic resonance hydrogen spectrum (1H-NMR, 400 MHz, CDCl3): δ 9.95 (s, 1H), 8.16 (s, 1H); nuclear magnetic resonance carbon spectrum (13C-NMR, 100 MHz, CDCl3): δ 180.93, 150.47, 145.48, 142.91; mass spectra (ESI): m/z 192.31 ([MH+]).

687636-93-7
118 suppliers
$24.00/250mg

464192-28-7
161 suppliers
$12.00/250mg
Yield:464192-28-7 91%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0 - 20; for 2 h;
Steps:
1 Step 1: 2-Bromothiazole-5 -carbaldehyde
Into a 500-mL round-bottom flask, was placed (2-bromothiazol-5-yl)methanol (20 g, 103 mmol), DCM (200 mL). This was followed by the addition of Dess-Martin reagent (46 g, 103 mmol) in portions at 0°C. The resulting solution was stirred for 2 h at RT and then was concentrated under vacuum. The residue was applied onto a silica gel column and eluted with ethyl acetate/petroleum ether (1:20 to 1:10). This resulted in 18 g (91%) of the title compound as awhite solid. MS-ESI: 193.9, 191.9 (M+1).
References:
IFM TRE, INC.;VENKATRAMAN, Shankar;GHOSH, Shomir;ROUSH, William R.;SHEN, Dong-Ming;KATZ, Jason;SEIDEL, Hans Martin WO2019/79119, 2019, A1 Location in patent:Page/Page column 462
1003-61-8
187 suppliers
$10.00/1g

464192-28-7
161 suppliers
$12.00/250mg