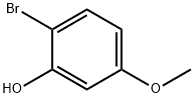
2-BROMO-5-METHOXYPHENOL synthesis
- Product Name:2-BROMO-5-METHOXYPHENOL
- CAS Number:63604-94-4
- Molecular formula:C7H7BrO2
- Molecular Weight:203.03
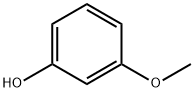
150-19-6

63604-94-4
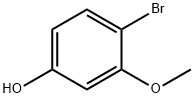
102127-34-4
40.0 g (322.2 mmol) of 3-methoxyphenol was dissolved in 1 L of acetonitrile and cooled to 0 °C under nitrogen protection. A solution of 57.35 g (322.2 mmol) of N-bromosuccinimide in 500 mL of acetonitrile was added slowly dropwise, with the rate of dropwise acceleration being controlled to maintain the temperature of the reaction mixture at 0 °C (for about 2 hours). After titration was completed, the reaction mixture was continued to be stirred at 0 °C for 1 hour. After completion of the reaction, the reaction mixture was concentrated under reduced pressure. The residue was treated with carbon tetrachloride and filtered to remove the resulting solid. The filtrate was concentrated under reduced pressure to give a mixture of brominated isomers in the form of a red oil. The mixture was separated by silica gel column chromatography using a hexane gradient elution system containing 0-30% ethyl acetate. The first eluted fractions were collected and concentrated under reduced pressure to give 18.1 g (28% yield) of 2-bromo-5-methoxyphenol as a clear liquid.1H-NMR (CDCl3): δ 7.31 (d, 1H), 6.6 (d, 1H), 6.41 (dd, 1H), 5.5 (s, 1H), 3.77 (s, 3H). Subsequently, the post-eluted fractions were combined and concentrated under reduced pressure. The residue was further purified by silica gel column chromatography using dichloromethane as eluent. The fraction containing high purity 4-bromo-3-methoxyphenol was collected and concentrated under reduced pressure to give 24.1 g (37% yield) of white crystalline solid (melting point 68-69 °C).1H-NMR (CDCl3): δ 7.34 (d, 1H), 6.45 (d, 1H), 6.33 (dd, 1H), 4.9 (br s, 1H), 3.85 (s, 3H).
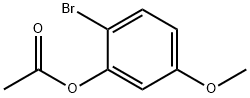
328945-89-7
1 suppliers
inquiry

63604-94-4
153 suppliers
$7.00/250mg
Yield: 93.4%
Reaction Conditions:
with water;sodium acetate in ethanol at 80; for 4 h;Temperature;
Steps:
3.3; 4.3; 5.3; 6.3
3) The result of step 2)2-bromo-5-methoxyphenyl acetate1.74 g (7.12 mmol) was put into a 100 mL three-necked flask, then 20.0 mL of ethanol was added to the flask, and finally 6.16 g (71.20 mmol) of sodium acetate and 15 mL of water were put into the flask under magnetic stirring; the water bath was heated to 80 ° C, 80 The reaction was stirred at for 4.0h.After the reaction, the reaction solution was acidified with 1 mol / L hydrochloric acid (about 17.4 mL) to a pH of 5,For the rest of the post-treatment, refer to step 3) of Example 1 to obtain 1.35 g of 2-bromo-5-methoxyphenol as pure white crystals with a yield of 93.4%.
References:
Hangzhou Maishiteng Pharmaceutical Technology Co., Ltd.;Jin Hao;Qian Chao;Gao Zhuo CN110317129, 2019, A Location in patent:Paragraph 0064; 0069-0071; 0076-0078; 0083-0085; 0090; 0091

150-19-6
366 suppliers
$6.00/5g

63604-94-4
153 suppliers
$7.00/250mg

150-19-6
366 suppliers
$6.00/5g

63604-94-4
153 suppliers
$7.00/250mg

102127-34-4
167 suppliers
$38.00/1g

150-19-6
366 suppliers
$6.00/5g

63604-94-4
153 suppliers
$7.00/250mg
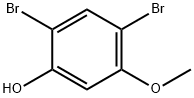
66693-87-6
2 suppliers
inquiry

102127-34-4
167 suppliers
$38.00/1g
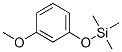
33285-71-1
0 suppliers
inquiry

63604-94-4
153 suppliers
$7.00/250mg

102127-34-4
167 suppliers
$38.00/1g