
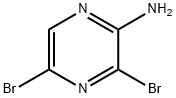
2-Bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine synthesis
- Product Name:2-Bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine
- CAS Number:1416740-16-3
- Molecular formula:C6H3BrN4O2
- Molecular Weight:243.02
24241-18-7
![2-Bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine](/CAS/20180531/GIF/1416740-16-3.gif)
1416740-16-3
Step 3: Synthesis of 2-bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine 2-Bromo-5H-pyrrolo[2,3-b]pyrazine (20 g, 101.0 mmol) was dissolved in pre-cooled concentrated sulfuric acid (140.0 mL) to form a bright orange solution. Concentrated nitric acid (12.73 g, 8.470 mL, 202.0 mmol) was slowly added dropwise over a period of 30 min under stirring conditions and ensuring that the temperature of the reaction was maintained below 10 °C (the color of the solution gradually changed to a clear red). After the dropwise addition was completed, the reaction mixture was removed from the ice bath and allowed to warm up slowly to room temperature, and stirring was continued at room temperature for 1 hour. Subsequently, the reaction mixture was slowly poured into crushed ice and a yellow solid was precipitated. The solid product was collected by filtration and washed well with cold water to afford 2-bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine as a yellow solid (21.76 g, 88.7% yield). Mass spectrometry analysis (ES+): m/z 244.87.

24241-18-7
439 suppliers
$6.00/5g
![2-Bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine](/CAS/20180531/GIF/1416740-16-3.gif)
1416740-16-3
26 suppliers
$51.00/100mg
Yield:1416740-16-3 88.7%
Reaction Conditions:
with sulfuric acid;nitric acid at 10 - 20; for 1.5 h;
Steps:
2.3
Step 3: 2-bromo-7-nitro-5H-pyrrolo[2,3-b]pyrazine[00199] 2-bromo-5H-pyrrolo[2,3-b]pyrazine (20 g, 101.0 mmol) was dissolved in ice-cold sulfuric acid (140.0 mL) , producing a bright orange solution, and concentrated nitric acid (12.73 g, 8.470 mL, 202.0 mmol) was added drop wise with stirring over 30 min keeping the temperature under 10 °C (turning the solution to a clear red colour). The reaction was removed from the ice bath after 30 min and allowed to warm to ambient temperature and left to stir at ambient temperature for 1 hour. The reaction mixture was poured onto ice to obtain a yellow solid. The solid was filtered, washed with water to give 2-bromo-7-nitro-5H- pyrrolo[2,3-b]pyrazine the desired product as a yellow solid. (21.76 g, 88.7%). MS (ES+) 244.87.
References:
WO2012/178123,2012,A1 Location in patent:Page/Page column 64