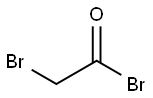
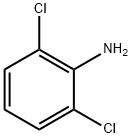
2-bromo-N-(2,6-dichlorophenyl)acetamide synthesis
- Product Name:2-bromo-N-(2,6-dichlorophenyl)acetamide
- CAS Number:32428-75-4
- Molecular formula:C8H6BrCl2NO
- Molecular Weight:282.95
Yield:32428-75-4 16%
Reaction Conditions:
with triethylamine at 0; for 1 h;Inert atmosphere;
Steps:
General procedure B: Synthesis of N-aryl bromoacetamides 12a-v
General procedure: To a solution of substituted aniline 11a-v (1 equiv) and triethylamine (1.1 equiv) in CH2Cl2 at 0 C was added bromoacetyl bromide (1 equiv) dropwise over 15 min and the mixture was stirred for 1 h at 0 C. The mixture was then diluted with CH2Cl2, washed with HCl x 2, water, sat. aqueous NaHCO3, brine, and dried (Na2SO4) before the solvent was removed in vacuo to give bromoacetamides 12a-v.
References:
Leung, Euphemia;Pilkington, Lisa I.;van Rensburg, Michelle;Jeon, Chae Yeon;Song, Mirae;Arabshahi, Homayon J.;De Zoysa, Gayan Heruka;Sarojini, Vijayalekshmi;Denny, William A.;Reynisson, Jóhannes;Barker, David [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 5,p. 1142 - 1154] Location in patent:supporting information