
2-BROMO-N-CYCLOPENTYLACETAMIDE synthesis
- Product Name:2-BROMO-N-CYCLOPENTYLACETAMIDE
- CAS Number:883521-80-0
- Molecular formula:C7H12BrNO
- Molecular Weight:206.08
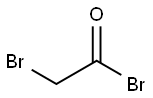
598-21-0
303 suppliers
$10.00/10g
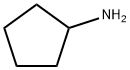
1003-03-8
210 suppliers
$10.00/1g

883521-80-0
10 suppliers
$60.00/10mg
Yield:883521-80-0 87%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 12 h;
Steps:
3.1.3. General Procedure for the Synthesis of Bromoacetamide Intermediate Derivatives
General procedure: In a first vial, dichloromethane (10 mL) was added to bromoacetyl bromide (216 L,2.48 mmol, 1 eq). In a second vial, dichloromethane (10 mL) was added to the correspondingamine (2.48 mmol, 1 eq) and Et3N (345 L, 2.48 mmol, 1 eq). The two reactions mixtureswere respectively stirred for 5 min at 0 C in a bath of ice. The second solution mixturewas added dropwise at 0 C to the first bromoacetyl bromide solution, and the reactionmixture was stirred from 0 C to room temperature for 12 h. The solution was poured intoa bath of ice. The aqueous layer was extracted with dichloromethane (3 100 mL) and theorganic layer was washed with brine (3 100 mL), dried over Na2SO4, and evaporated.Intermediate compounds were obtained without purification, except for compounds 7, 10,11, 14.
References:
Brun, Damien;Di Giorgio, Carole;Kabri, Youssef;Mathias, Fanny;Primas, Nicolas;Vanelle, Patrice [Molecules,2022,vol. 27,# 7,art. no. 2163] Location in patent:supporting information
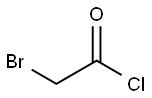
22118-09-8
172 suppliers
$21.21/10gm:

1003-03-8
210 suppliers
$10.00/1g

883521-80-0
10 suppliers
$60.00/10mg