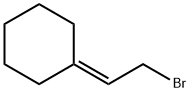
(2-bromoethylidene)cyclohexane synthesis
- Product Name:(2-bromoethylidene)cyclohexane
- CAS Number:932-86-5
- Molecular formula:C8H13Br
- Molecular Weight:189.09
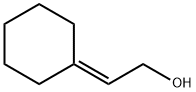
932-89-8
15 suppliers
inquiry

932-86-5
6 suppliers
inquiry
Yield:932-86-5 99%
Reaction Conditions:
with phosphorus tribromide in diethyl ether at 0;Inert atmosphere;
Steps:
1 Allylic Bromide 47
Allylic Bromide 47
To a stirred solution of alcohol 46 (7.0 g, 55.5 mmol) in diethyl ether (70 mL) under N2 at 0° C. was added phosphorous tribromide (1.71 mL, 18.51 mmol) drop wise over 10-15 min.
The resulting reaction mixture was stirred at 0° C. for additional hour, which was followed by TLC.
After completion of the reaction progress, it was quenched with water (10 mL), the diethyl ether was removed under a reduced pressure and the oily residue was diluted with water (200 mL).
The aqueous material was then extracted with n-hexanes (3*?200 mL), the combined hexanes were washed with brine (150 mL) dried over anhydrous MgSO4 and concentrated under a reduced pressure to afford the desired bromide 47 (10.4 g, 99%) which was used as such in the next step without purification to prepare the ketoesters 48.
References:
US2013/85283,2013,A1 Location in patent:Paragraph 0280
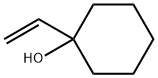
1940-19-8
26 suppliers
$182.00/5g

932-86-5
6 suppliers
inquiry
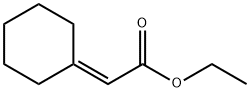
1552-92-7
76 suppliers
$18.00/1g

932-86-5
6 suppliers
inquiry
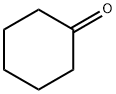
108-94-1
583 suppliers
$12.00/50g

932-86-5
6 suppliers
inquiry
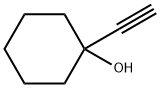
78-27-3
378 suppliers
$8.00/25g

932-86-5
6 suppliers
inquiry