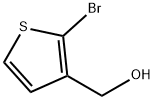
(2-Bromothien-3-yl)methanol synthesis
- Product Name:(2-Bromothien-3-yl)methanol
- CAS Number:70260-16-1
- Molecular formula:C5H5BrOS
- Molecular Weight:193.06
71637-34-8

70260-16-1
General procedure for the synthesis of (2-bromo-3-thiophene) methanol from 3-thiophene methanol: 3-hydroxymethylthiophene (12, 4.06 g, 35.6 mmol) was dissolved in 18 mL of acetic acid at room temperature, followed by the addition of N-bromosuccinimide (6.34 g, 35.6 mmol), and the reaction was stirred for 45 min. After completion of the reaction, the reaction was quenched with 1 mL of water and the mixture was poured into 250 mL of ether and washed sequentially with 100 mL of water and 100 mL of 10% aqueous NaHCO3. The organic layer was separated, dried with anhydrous Na2SO4 and the solvent was concentrated. Finally, 6.25 g of crude product 13 was obtained in 91% yield, which was directly used in the next reaction without further purification.

71637-34-8
229 suppliers
$5.00/1g

70260-16-1
64 suppliers
$78.00/250mg
Yield:70260-16-1 91%
Reaction Conditions:
with NBS;glacial acetic acid at 20; for 0.75 h;
Steps:
8 2-BROMO-3-HYDROXYMETHYLTHIOPHENE (13)
To a solution of 3-hydroxymethylthiophene 12 (4.06 g, 35.6 mmol) in 18 mL of acetic acid was added N-bromosuccinimide (6.34 g, 35.6 mol) at room temperature and stirred for 45 minutes. The resulting mixture was quenched with 1 mL of water, poured into 250 mL ether and washed with 100 mL of water, 100 mL of 10% NAHC03 aq. respectively. The organic layer was dried over NA2S04 and concentrated. 6.25 G of crude product 13 (91 %) was obtained and used for the subsequent reaction without further purification.
References:
WO2004/65384,2004,A1 Location in patent:Page 36; 16/19
40032-76-6
126 suppliers
$20.00/250mg

70260-16-1
64 suppliers
$78.00/250mg

189331-42-8
0 suppliers
inquiry

70260-16-1
64 suppliers
$78.00/250mg
616-44-4
253 suppliers
$18.00/25g

70260-16-1
64 suppliers
$78.00/250mg