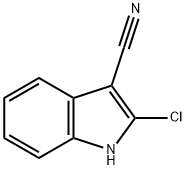
2-Chloro-1H-indole-3-carbonitrile synthesis
- Product Name:2-Chloro-1H-indole-3-carbonitrile
- CAS Number:156136-56-0
- Molecular formula:C9H5ClN2
- Molecular Weight:176.6
5059-30-3
118 suppliers
$20.00/100mg

156136-56-0
11 suppliers
inquiry
Yield:156136-56-0 58%
Reaction Conditions:
with pyridine in Ac2 O;ethanol;water;
Steps:
I Compound 120 of Table 1
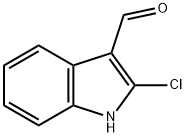
Compound 120 of Table 1 A mixture of 2-chloroindole-3-carboxaldehyde (7.0 g, 36 mmol) was reacted with a slight excess of hydroxylamine hydrochloride and pyridine in refluxing EtOH for 1 hour, to give the crude oxime (Latrell R, Bartmann W, Musif J, Granzer E, German Patent 2,707,268, 31 Aug 1978, Chem. Abstr. 1978;89:179858y). A solution of this in Ac2 O (100 mL) was heated under reflux for 1 hour, cooled, and stirred with water (700 mL). The precipitated solid was collected, washed with water, and crystallized from aqueous MeOH to give 2-chloro-1H-indole-3-carbonitrile (3.7 g, 58%); mp 177°-180° C. 1 H NMR ((CD3)2 SO): δ13.23 (1H, s, NH), 7.60 (1H, d, J=7.5 Hz, ArH), 7.50 (1H, d, J=7.9 Hz, ArH), 7.34 (1H, t, J=7.5 H, ArH), 7.29 (1H, t, J=7.3 Hz, ArH). 13 C NMR: δ134.0, 131.5, 126.2, 114.1 (C), 123.8, 122.3, 117.9, 112.3 (CH), 83.8 (CN). Analysis calculated for C9 H5 ClN2 requires: C, 61.2; H, 2.9; N, 15.9%. Found: C, 61.2; H, 2.7; N, 15.9%.
References:
US5464861,1995,A

1269407-11-5
1 suppliers
inquiry

156136-56-0
11 suppliers
inquiry